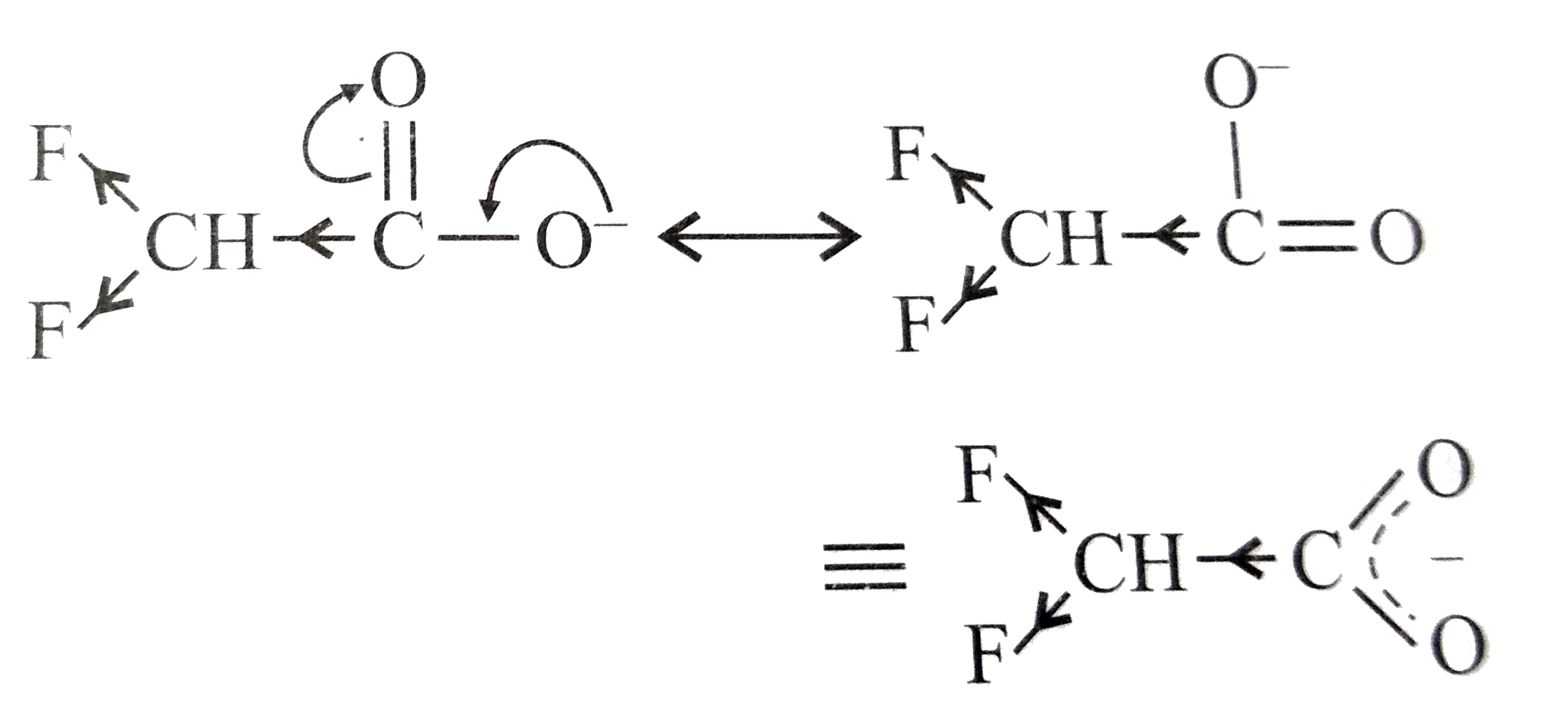
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES AND TECHNIQUES
NCERT FINGERTIPS ENGLISH|Exercise Assertion & Reason|13 VideosORGANIC CHEMISTRY-SOME BASIC PRINCIPLES AND TECHNIQUES
NCERT FINGERTIPS ENGLISH|Exercise Tetravalence Of Carbon : Shape Of Organic Compounds|5 VideosORGANIC CHEMISTRY-SOME BASIC PRINCIPLES AND TECHNIQUES
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|12 VideosHYDROGEN
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosPRACTICE PAPER 1
NCERT FINGERTIPS ENGLISH|Exercise Practice Paper 1|44 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES AND TECHNIQUES -Exemplar Problems
- Which of the following is the correct IUPAC name?
Text Solution
|
- The IUPAC name for CH(3)-overset(O)overset(||)(C)-CH(2)-CH(2)-overset(...
Text Solution
|
- The IUPAC name for
Text Solution
|
- Electronegativity of carbon atoms depends upon their state of hybridis...
Text Solution
|
- In which of the followinng functional group isomerism is not possible?
Text Solution
|
- The fragrance of flowers is due to the presence of some steamm volatil...
Text Solution
|
- During hearing of a court case, the judge suspected that some changes ...
Text Solution
|
- The principle involved in paper chromatography is
Text Solution
|
- What is the correct order of decreasing stability of the following cat...
Text Solution
|
- In which of the followinng compounds the carbon marked with asterisk i...
Text Solution
|
- Ionic species are stabilised by the dispersal of charge. Which of the ...
Text Solution
|
- Electrophilic addition reactions proceed in two steps. The first step ...
Text Solution
|