A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-PRACTICE PAPER 1-Practice Paper 1
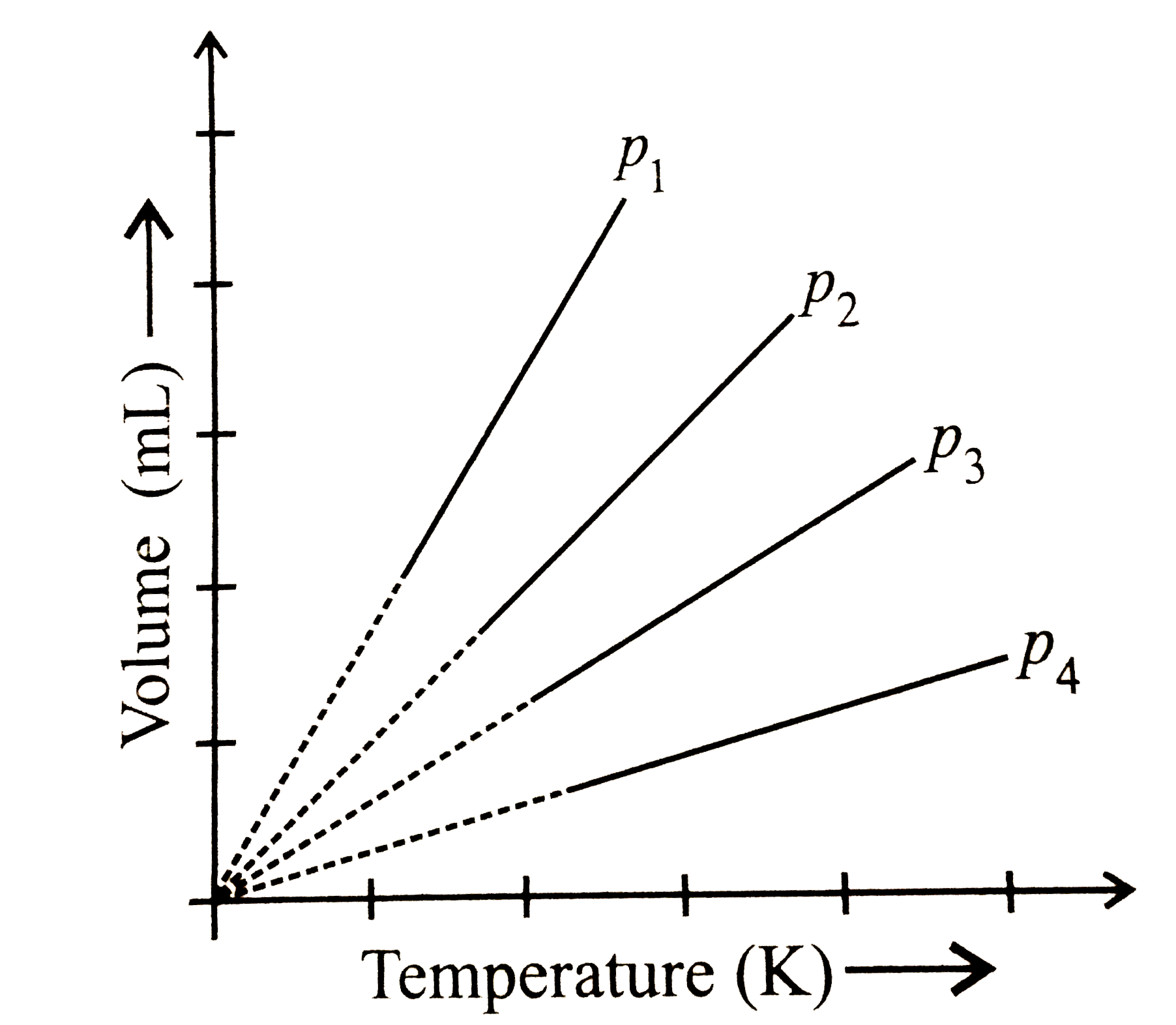
- A plot of volume (V) versus temperature (T) for a gas at constannt pre...
Text Solution
|
- The equilibrium constant at 717 K for the reaction: H(2(g))+I(2(g))lAr...
Text Solution
|
- Which of the following statements is not correct?
Text Solution
|
- Which one of the following pairs do not impart colour to the flame?
Text Solution
|
- Which is not correct?
Text Solution
|
- aK(2)Cr(2)O(7)+bKCl+cH(2)SO(4) to xCrO(2)Cl(2)+yKHSO(4)+zH(2)O The a...
Text Solution
|
- Which of the following is correct for SF(4)?
Text Solution
|
- The strongest bond is present in
Text Solution
|
- Carbon and oxygen form two compounds. Carbon content in one of them is...
Text Solution
|
- For the reaction at 25^(@)C,X(2)O(4(l))to2XO(2(g)),DeltaH=2.1kcal and ...
Text Solution
|
- Which of the following statements is incorrect?
Text Solution
|
- Which of the following statements is correct?
Text Solution
|
- Which of the following is wrong?
Text Solution
|
- The central C-atom of a carbanion possesses
Text Solution
|
- Indusstrially, H(2)O(2) is obtainned by the following cyclic process: ...
Text Solution
|
- The amont of water produced by the combustion of 16 g of methane is
Text Solution
|
- The order of heat of fusion of T(2),D(2) and H(2) is
Text Solution
|
- The photochemical smog is essentially caused by presence by
Text Solution
|
- Which of the following orbital overlapping is not possible according t...
Text Solution
|
- The reaction, RC-=CR underset("Lindlar's catalyst")overset(H(2))toGive...
Text Solution
|
- Which statement is false?
Text Solution
|