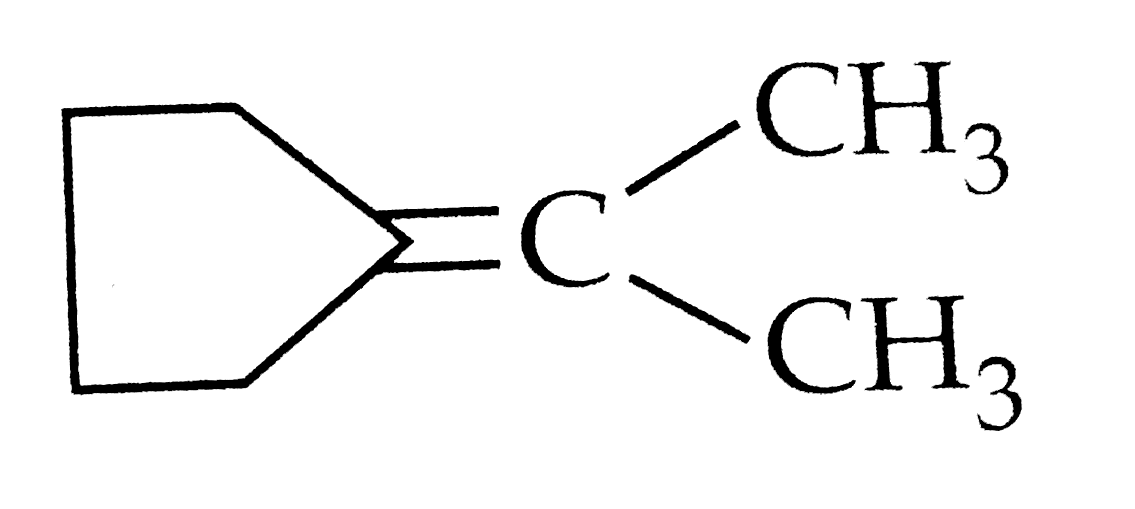
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-PRACTICE PAPER 1-Practice Paper 1
- The compressibility of a gas is less than unity at STP. Therefore,
Text Solution
|
- Element M+N(2) overset(Delta)to overset(H(2)O)toNH(3) element M belong...
Text Solution
|
- Select the correct statement. In the gas equation, PV=nRT
Text Solution
|
- The solubility product of aluminium sulphate is given by the expressio...
Text Solution
|
- The IUPAC name of the compound, underset(OH)underset(|)(C)H(2)-underse...
Text Solution
|
- Calculate the uncertainty in the momentum of an electron if it is conf...
Text Solution
|
- The second ionization enthalpy is
Text Solution
|
- IUPAC name of 4-iso-propyl-m-xylene is
Text Solution
|
- The positive value of DeltaS indicates that
Text Solution
|
- Capilary action of the liquid can be explained on the basis of its
Text Solution
|
- Smoke is an example of
Text Solution
|
- The first emission line in the atomic spectrum of hydrogen in the Balm...
Text Solution
|
- The ratio of specific charge of a proton and an alpha-particle is
Text Solution
|
- Photochemical smog is in character while classical smog is in characte...
Text Solution
|
- Which of the following is sparingly soluble in water?
Text Solution
|
- Two members of a homologous series have different
Text Solution
|
- Free energy change for a reversible process is
Text Solution
|
- Which of the following ions is smallest in size?
Text Solution
|
- The bond angle H-O-H in ice is closest to
Text Solution
|
- Which of the following alkenes on ozonolysis gives a mixture of ketone...
Text Solution
|