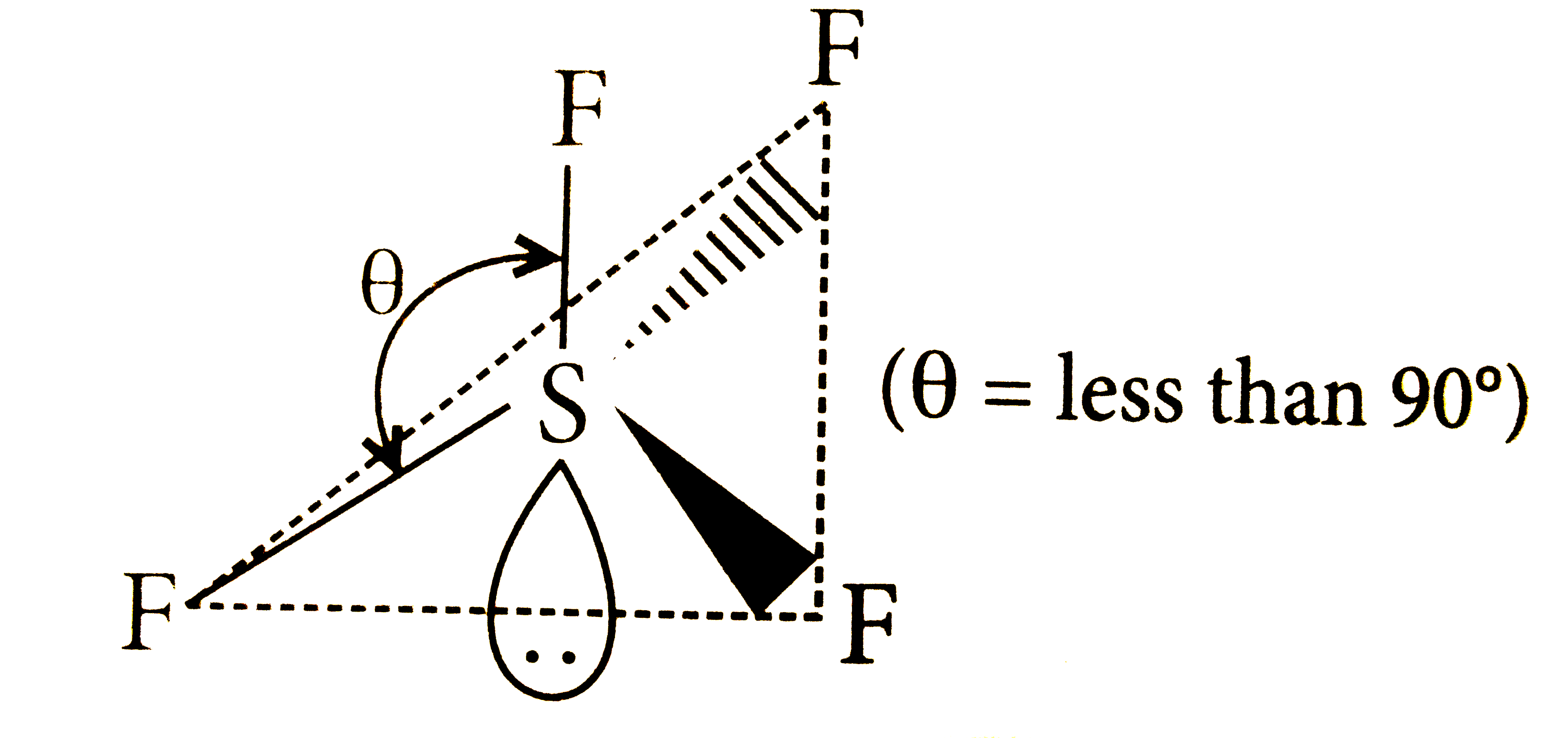
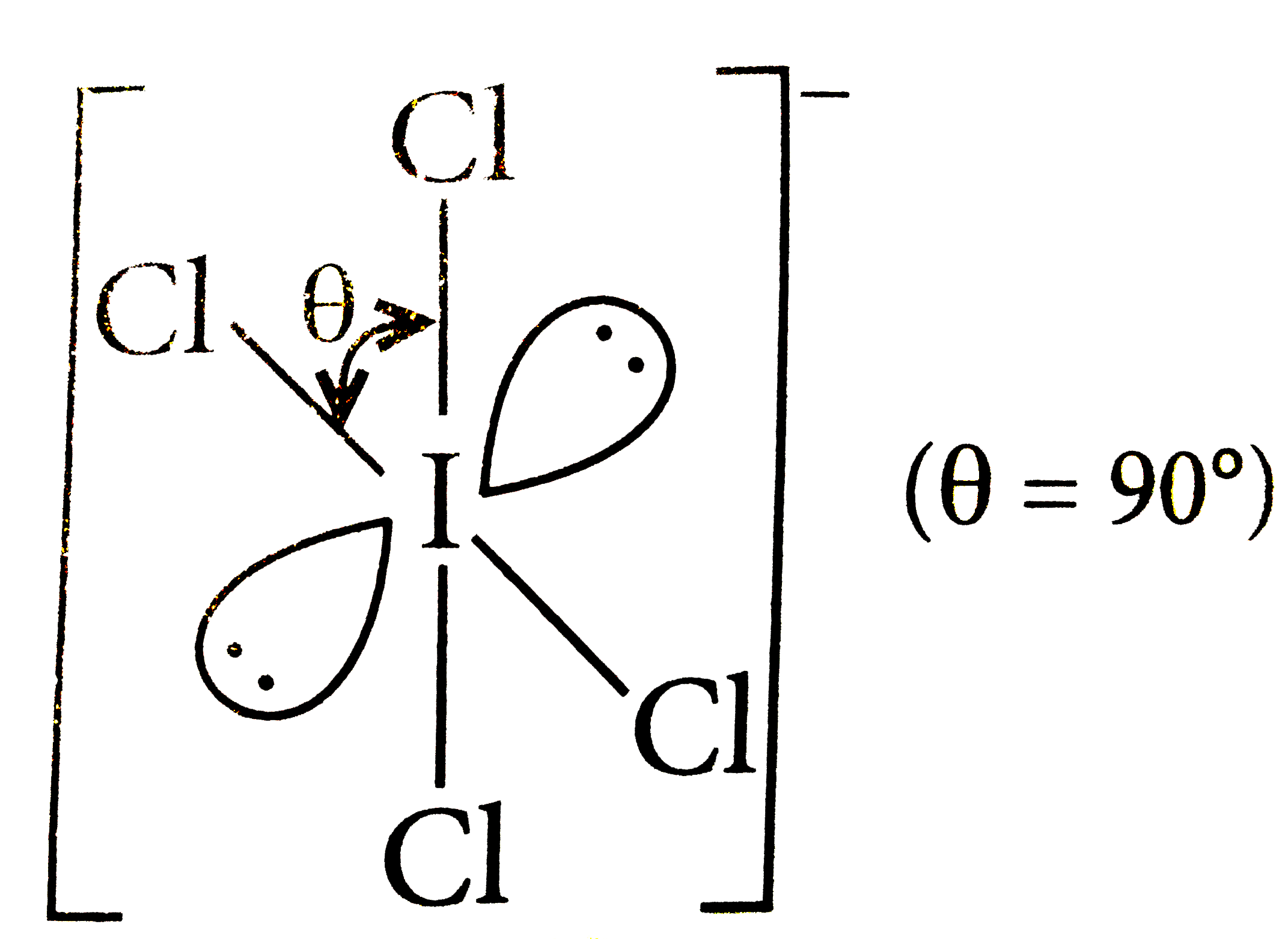
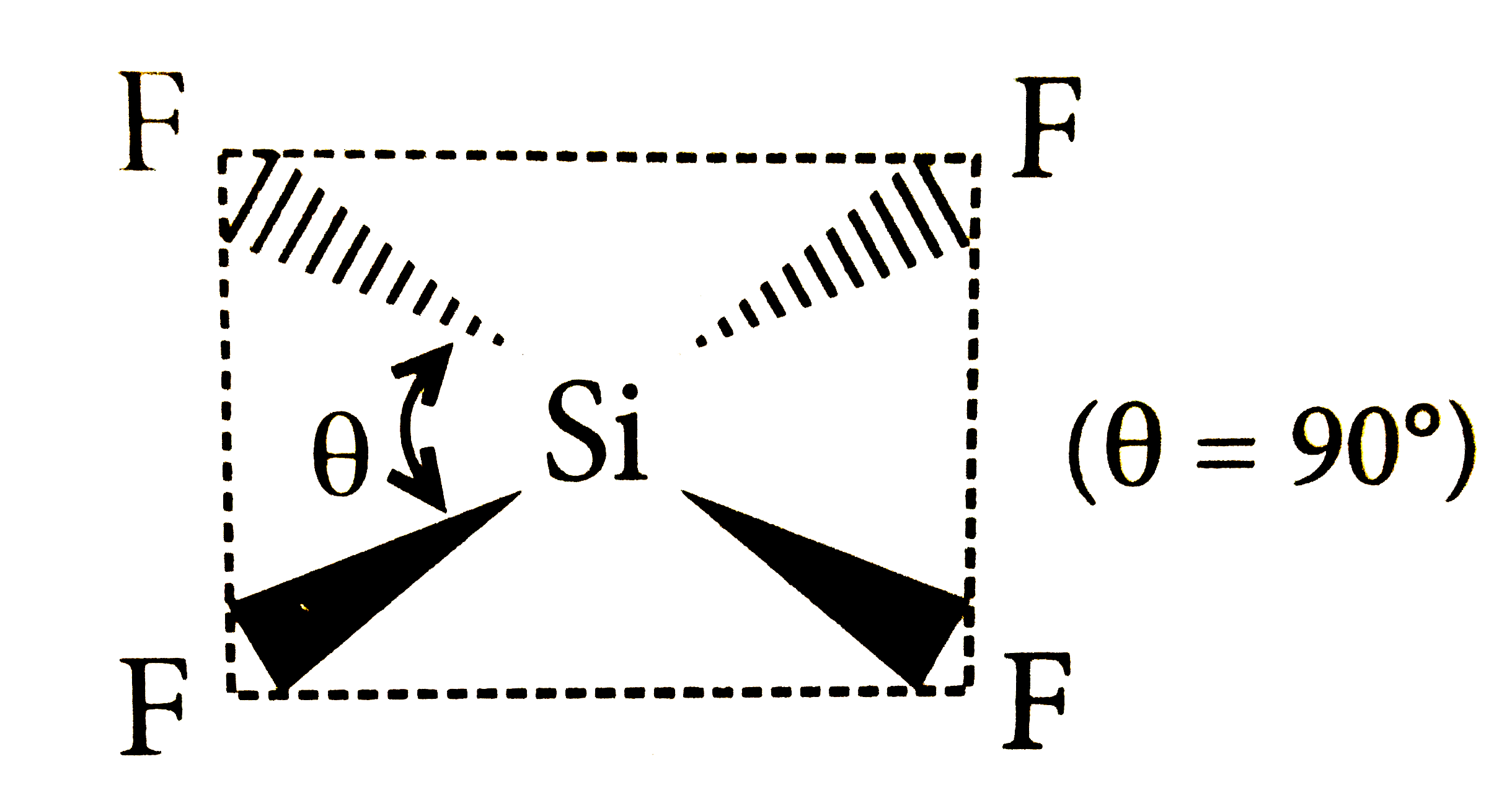
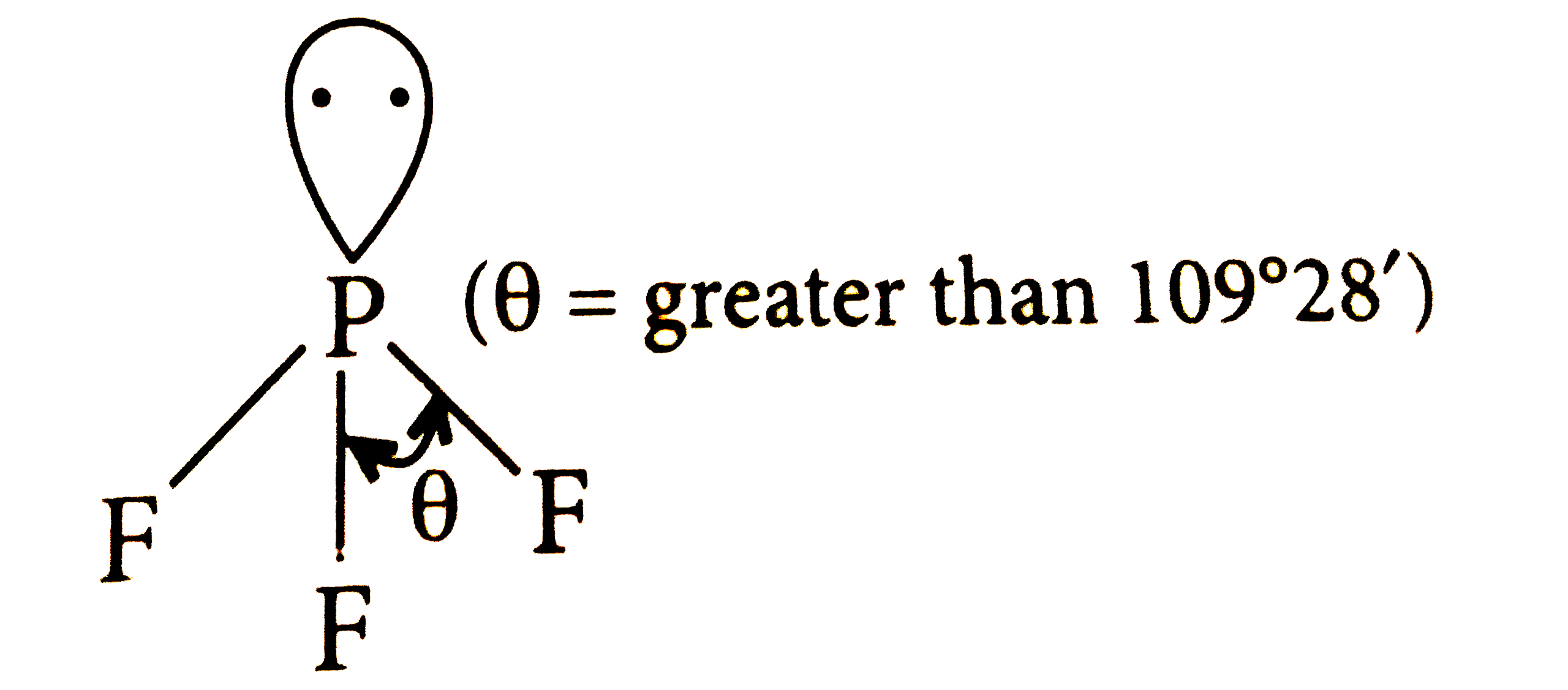A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-PRACTICE PAPER 2-Practice Paper 2
- The bond order of a molecule is given by
Text Solution
|
- Permanent hardness is due to presence of soluble salts of Mg and Ca in...
Text Solution
|
- Mark out the correct increasing order of radius.
Text Solution
|
- DeltaH("neutralisation") is always
Text Solution
|
- The pH of blood is
Text Solution
|
- Which of the following is according to Boyle's law?
Text Solution
|
- pH of a 1.0xx10^(-8) M solution of HCl is
Text Solution
|
- The substance used as a adsorbentt in the column
Text Solution
|
- Elements of group 14 exhibit oxidation state of
Text Solution
|
- With rise in temperature, viscosity of a liquid
Text Solution
|
- Air contains 21% of oxygen by volume. The number of moles of O(2) pres...
Text Solution
|
- The ratio of average speed of an oxygen molcule to the RMS speed of a ...
Text Solution
|
- The kinetic enerrgy of 4 mole sof nitrogen gas at 127^(@)C is (R=2" ca...
Text Solution
|
- Out off N(2)O,SO(2),I(3)^(+),I(3)^(-),H(2)O,NO(2)^(-),N(3)^(-), the li...
Text Solution
|
- In which of the following ionisation processes, the bond order has inc...
Text Solution
|
- Which of the following structure is correctly drawn according to funda...
Text Solution
|
- Back bonding in BF(3) does not afect
Text Solution
|
- Ammonium carbamate when heated tto 200^(@)C gives a mixture of NH(3) a...
Text Solution
|
- For the reaction, A((g))+2B((g))hArr 3C((g))+3((g)),K(p)=0.05" atm at ...
Text Solution
|
- Consider the following reaction, (i) CO(3)^(2-)+H(2)OhArrHCO(3)^(-)+...
Text Solution
|