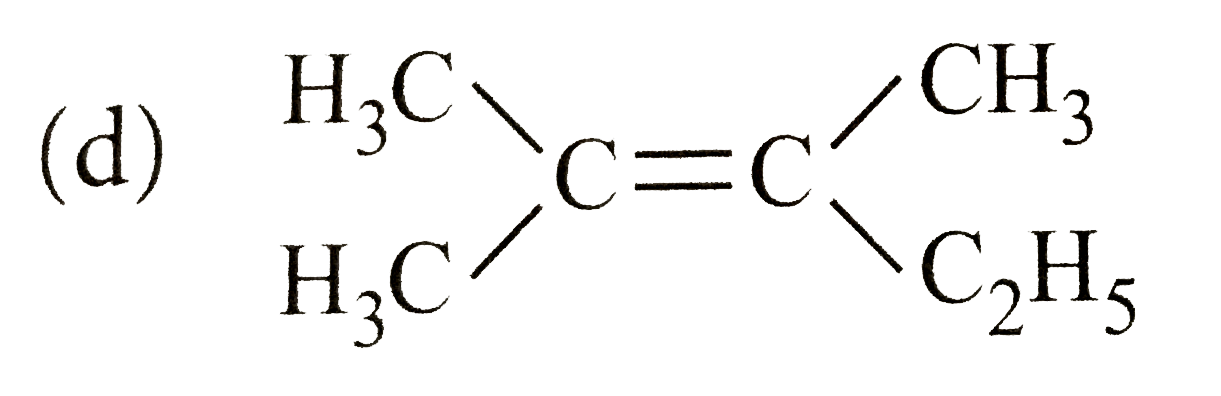
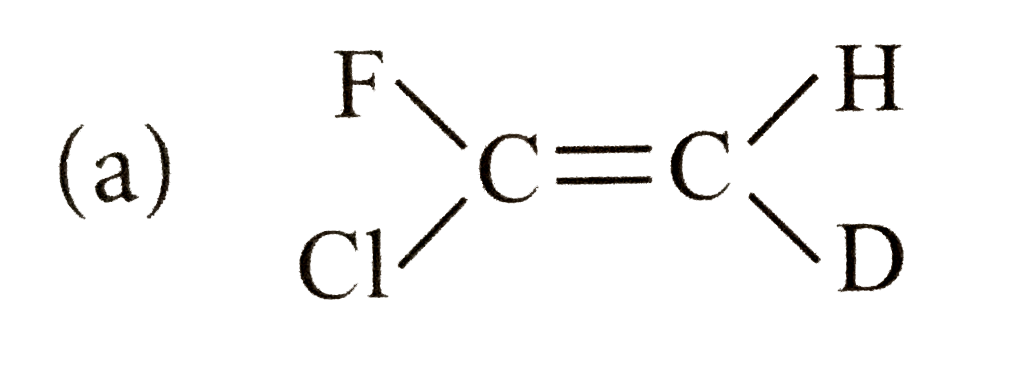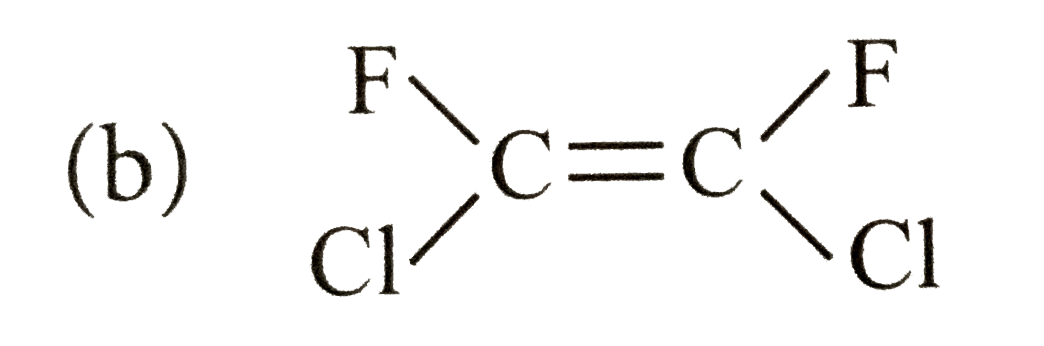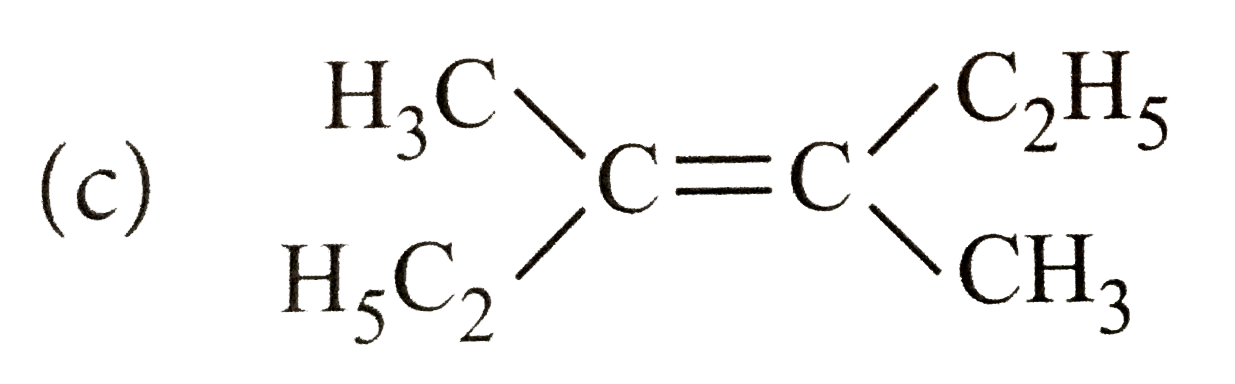A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-HYDROCARBONS -Exemplar Problems
- Arrange the following in decreasing order of their boiling points: (...
Text Solution
|
- Arrange the halogens F(2),Cl(2),Br(2),I(2), in order of their increasi...
Text Solution
|
- The increasing order of reduction of alkyl halides with zinc and dilut...
Text Solution
|
- The correct IUPAC name of the following alkane is
Text Solution
|
- The addition off HBr to 1-butene gives a mixture of products (I), (II)...
Text Solution
|
- Which of the following will not show geometrical isomerism?
Text Solution
|
- Arrange the followingg hydrogen halides in order of their decreasin re...
Text Solution
|
- Arrange the following carbanions in order of their decreasing stabilit...
Text Solution
|
- Arrange the following alkyl halides in decreasing order of the rate of...
Text Solution
|
- Which of the followingg reactions of methane is incomplete combustion?
Text Solution
|