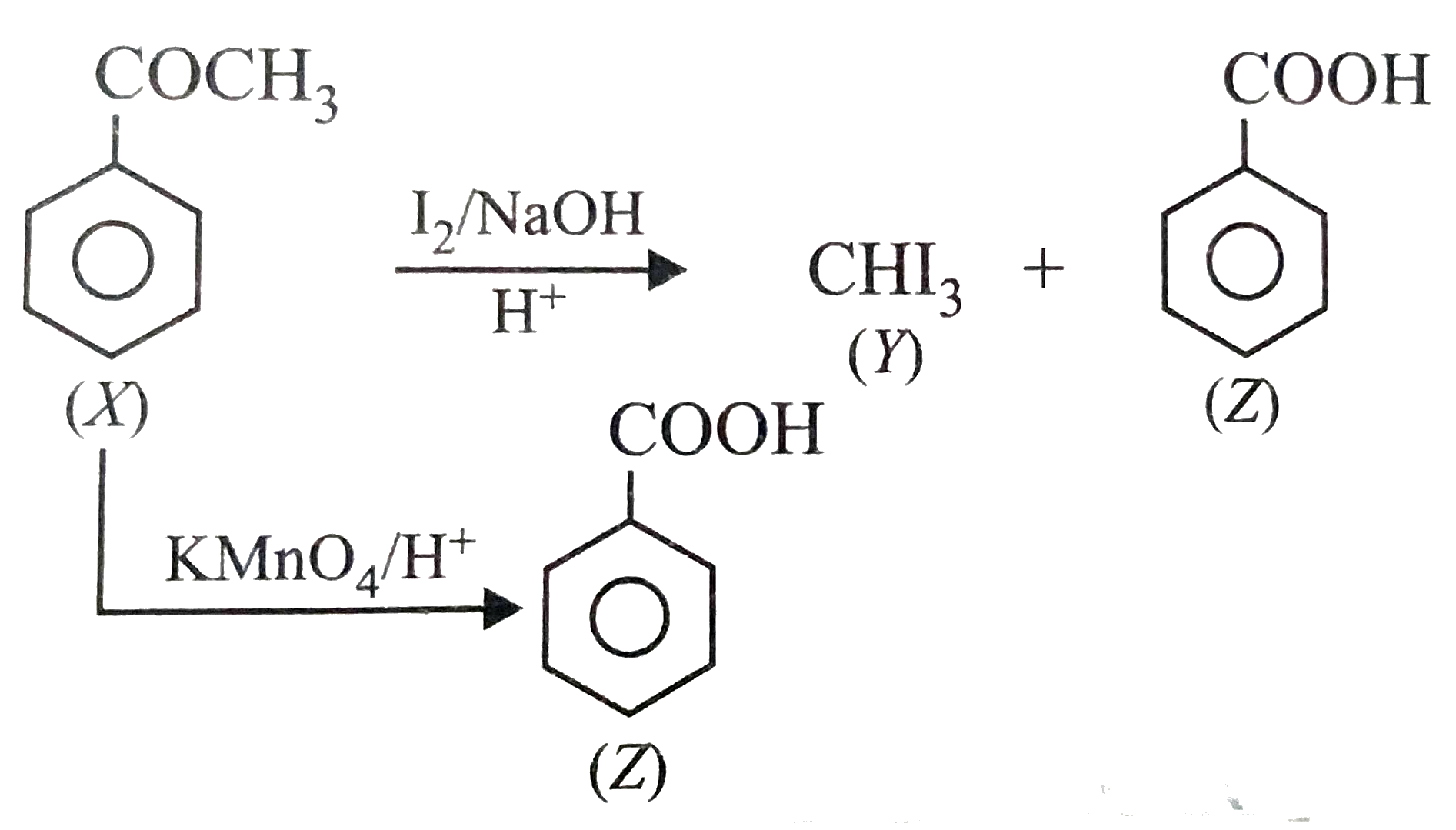
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES, KETONES AND CARBOXYLIC ACIDS
NCERT FINGERTIPS ENGLISH|Exercise MCQs( PHYSICAL PROPERTIES )|2 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
NCERT FINGERTIPS ENGLISH|Exercise MCQs(CHEMICAL REACTIONS )|18 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
NCERT FINGERTIPS ENGLISH|Exercise MCQs( NOMENCLATURE AND STRUCTURE OF CARBOXYL GROUP )|2 VideosALCOHOLS,PHENOLS AND ETHERS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|14 VideosAMINES
NCERT FINGERTIPS ENGLISH|Exercise ASSERTION & REASON CORNER|14 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-ALDEHYDES, KETONES AND CARBOXYLIC ACIDS-MCQs( METHODS OF PREPARATION OF CARBOXYLIC ACIDS )
- Which of the following reactions does not occur ?
Text Solution
|
- Which of the following will not yield acetic acid on strong oxidation ...
Text Solution
|
- Various products formed on oxidation of 2, 5-dimethylhexan-3-one are ...
Text Solution
|
- alpha-hydroxypropanoic acid can be prepared from ethanal by following ...
Text Solution
|
- The end product (Z) in the given sequence of reaction is CH(3)CH=CHC...
Text Solution
|
- Complete the reactions with appropriate products. CH(3)CHO+NH(2)OH t...
Text Solution
|
- An aromatic compound (X) (C(8)H(8)O) gives positive 2, 4-DNP test. It ...
Text Solution
|