Text Solution
Verified by Experts
Topper's Solved these Questions
ACIDS,BASES AND SALTS
OSWAAL PUBLICATION|Exercise Topic-2 MCQ|2 VideosACIDS,BASES AND SALTS
OSWAAL PUBLICATION|Exercise Topic-2 Very Short Answer Type Questions|6 VideosACIDS,BASES AND SALTS
OSWAAL PUBLICATION|Exercise TOPIC -1 SHORT ANSWER TYPE QUESTIONS -II|17 VideosCARBON AND ITS COMPOUNDS
OSWAAL PUBLICATION|Exercise NCERT Corner (Textbook Exercises)|15 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-ACIDS,BASES AND SALTS -TOPIC-1 LONG ANSWER TYPE QUESTIONS
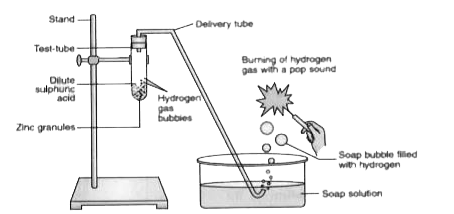
- In the following schematic diagram for the preparation of hydrogen gas...
Text Solution
|
- Define pH scale. Draw a figure showing variation of pH with the change...
Text Solution
|
- (i) Define universal indicator. For what purpose it is used? (ii) Tw...
Text Solution
|
- Account for the following: (i) State the relation between hydrogen i...
Text Solution
|
- (i) Acids as well as bases ionize in water . Name the ions produced by...
Text Solution
|
- You have four solutions A, B, C and D. The pH of solution A is 6, B is...
Text Solution
|
- In a tabular form write the colours of the following indicators in pre...
Text Solution
|
- (i) A local magician was showing magic in a village street. He took eg...
Text Solution
|
- (i) Name the gas which is liberated when an acid reacts with a metal. ...
Text Solution
|
- (i) Bee sting leaves a chemical substances that causes pain and irriga...
Text Solution
|
- (i) Write balanced chemical equations only for the following chemical ...
Text Solution
|