A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
ATOMIC STRUCTURE
MTG-WBJEE|Exercise WB JEE WORKOUT (CATEGORY 3 : One or More than One Option Correct Type (2 Marks))|12 VideosATOMIC STRUCTURE
MTG-WBJEE|Exercise WB JEE Previous Years Questions (CATEGORY 1 : Single Option Correct Type (1 Mark))|9 VideosATOMIC STRUCTURE
MTG-WBJEE|Exercise WB JEE Previous Years Questions (CATEGORY 2 : Single Option Correct Type (2 Marks)|1 VideosAROMATIC COMPOUNDS
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS (CATEGORY 2 : One Or More Option Correct Type)|4 VideosATOMS MOLECULES AND CHEMICAL ARITHEMETIC
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS (CATEGORY 2: SINGLE OPTION CORRECT TYPE (2 MARKS))|3 Videos
Similar Questions
Explore conceptually related problems
MTG-WBJEE-ATOMIC STRUCTURE-WB JEE WORKOUT (CATEGORY 2 : Single Option Correct Type (2 Marks))
- The ratio of the difference in energy between the first and the second...
Text Solution
|
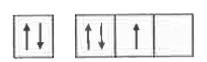
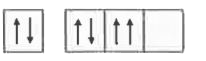
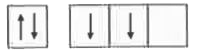
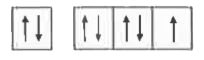
- The orbital diagram in which both the Pauli 's exclusion principle and...
Text Solution
|
- If electron, hydrogen, helium and neon nuclei are all moving with the ...
Text Solution
|
- Which one of the following set of quantum numbers is not possible for ...
Text Solution
|
- The nucleus of an atom can be assumed to be spherical. The radius of t...
Text Solution
|
- Assuming Rydberg constant (R(H)) to be 109670 cm^(- 1), the longest wa...
Text Solution
|
- Light of wavelength lamda shines on a metal surface with intensity x a...
Text Solution
|
- A particle A moving with a certain velocity has a de Broglie wavelengt...
Text Solution
|
- If n and l are respectively the principal and azimuthal quantum number...
Text Solution
|
- Which of the following element outermost orbit's last electron has mag...
Text Solution
|
- The value of Planck's constant is 6.63 xx 10^(-34) Js. The velocity of...
Text Solution
|
- In Bohr series of lines of hydrogen spectrum, the third line from the ...
Text Solution
|
- In the Bohr's orbit, what is the ratio of total kinetic energy and the...
Text Solution
|
- The frequency of the radiation emitted when the electron falls from n ...
Text Solution
|