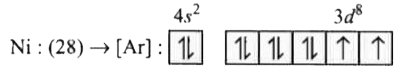A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
ATOMIC STRUCTURE
MTG-WBJEE|Exercise WB JEE Previous Years Questions (CATEGORY 2 : Single Option Correct Type (2 Marks)|1 VideosATOMIC STRUCTURE
MTG-WBJEE|Exercise WB JEE WORKOUT (CATEGORY 3 : One or More than One Option Correct Type (2 Marks))|12 VideosAROMATIC COMPOUNDS
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS (CATEGORY 2 : One Or More Option Correct Type)|4 VideosATOMS MOLECULES AND CHEMICAL ARITHEMETIC
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS (CATEGORY 2: SINGLE OPTION CORRECT TYPE (2 MARKS))|3 Videos
Similar Questions
Explore conceptually related problems
MTG-WBJEE-ATOMIC STRUCTURE-WB JEE Previous Years Questions (CATEGORY 1 : Single Option Correct Type (1 Mark))
- The emission spectrum of hydrogen discovered first and the region of t...
Text Solution
|
- As per de Broglie's formula a macroscopic patiicle of mass 100 g and m...
Text Solution
|
- The electronic configuration of Cu is
Text Solution
|
- The energy required to break one mole of hydrogen hydrogen bonds in H(...
Text Solution
|
- Which one of the following corresponds to a photon of highest energy?
Text Solution
|
- If the given four electronic configurations (i) n = 4, l = 1 (ii) n = ...
Text Solution
|
- Which of the following sets of quantum numbers represents the 19^("th"...
Text Solution
|
- Which of the following electronic configuration is not possible?
Text Solution
|
- The number of unpaired electrons in Ni (atomic number = 28) are
Text Solution
|