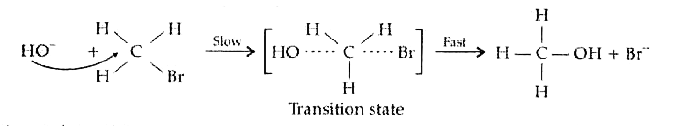
Text Solution
Verified by Experts
Topper's Solved these Questions
HALOALKANES & HALOARENES
OSWAAL PUBLICATION|Exercise Topic 2 (Properties of Haloarenes and Haloalkanes) (Long Answer Type Questions - II)|1 VideosHALOALKANES & HALOARENES
OSWAAL PUBLICATION|Exercise Topic 2 (Properties of Haloarenes and Haloalkanes) (Short Answer Type Questions)|14 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC 2 (LONG ANSWER TYPE QUESTIONS-II)|2 VideosII PUC ANNUAL EXAMINATION 2019
OSWAAL PUBLICATION|Exercise PART - D|10 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-HALOALKANES & HALOARENES-Topic 2 (Properties of Haloarenes and Haloalkanes) (Long Answer Type Questions - I)
- Identify the products A, B and C in the following equation. CH(3)OH ...
Text Solution
|
- Halo arenes are less reactive towards nucleophilic substitution reacti...
Text Solution
|
- Explain S(N) - 2 reaction mechanism ?
Text Solution
|
- Mention the major product formed by the following reacton: (a) 2- "B...
Text Solution
|
- Compete the following reaction. CH(3) underset(Z)underset(underset(H...
Text Solution
|
- Identify A and B in the following :
Text Solution
|