Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY
KUMAR PRAKASHAN|Exercise SECTION-A (Try Yourself)|25 VideosELECTROCHEMISTRY
KUMAR PRAKASHAN|Exercise SECTION-A (Practice Questions)|3 VideosELECTROCHEMISTRY
KUMAR PRAKASHAN|Exercise SECTION-E MCQ.s Asked in GUJCET/Board Exam|55 VideosCHEMISTRY IN EVERYDAY LIFE
KUMAR PRAKASHAN|Exercise SECTION - E (MCQs ASKED IN GUJCET/BOARD EXAM)|37 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION - E (MCQs ASKED IN GUJET/BOARD EXAMS)|46 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-ELECTROCHEMISTRY-SECTION-A
- Explain Kohlrausch law of independent migration of ions.
Text Solution
|
- Write Kohlrausch law and give its importance (applications).
Text Solution
|
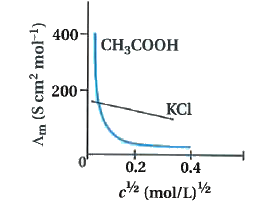
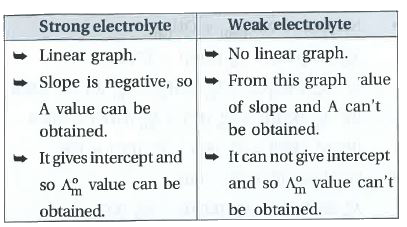
- Give graph of Lamda(m) to c^(1//2) of aqueous solution strong and weak...
Text Solution
|
- What is electrolyte cell ? Give its uses.
Text Solution
|
- Which is the simplest electrolytic cell ? Give detailed note on it.
Text Solution
|
- Give Faraday law and its uses.
Text Solution
|
- What is the use of coulometer ?
Text Solution
|
- Now a days, how quantity of electricity Q can be measured in electroly...
Text Solution
|
- Clarify stoichiometry of reaction occurs on the electrodes of electrol...
Text Solution
|
- "Products obtained near the electrodes by electrolysis depends on the ...
Text Solution
|
- Exalain: Reduction reaction is possible with higher E^(Theta) value. ...
Text Solution
|
- Give electrolytic reactions of molten NaCl occurrred in inert electrod...
Text Solution
|
- Explain : Oxidation of Cl^(-) ion is carried out near anode when elect...
Text Solution
|
- Give electrolytic reaction of aqueous NaCl solution (concentrated) in ...
Text Solution
|
- Give reaction occurred near anode of electrolysis of sulphuric acid in...
Text Solution
|
- Products of electrolysis depends upon which factors? Justify with suit...
Text Solution
|
- Give basic information on Battery.
Text Solution
|
- What is primary battery ? Give note on primary battery. OR Give note...
Text Solution
|
- Write a note on mercury cell (Zn-Hg).
Text Solution
|
- What is secondary cell ? Give examples of it.
Text Solution
|