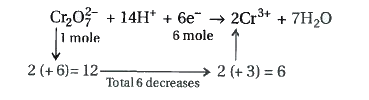Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY
KUMAR PRAKASHAN|Exercise SECTION-C Textual Exercise|14 VideosELECTROCHEMISTRY
KUMAR PRAKASHAN|Exercise SECTION-D NCERT Exemplar Solution (MCQ.s)|17 VideosELECTROCHEMISTRY
KUMAR PRAKASHAN|Exercise SECTION-A (Extra Examples for Practice)|2 VideosCHEMISTRY IN EVERYDAY LIFE
KUMAR PRAKASHAN|Exercise SECTION - E (MCQs ASKED IN GUJCET/BOARD EXAM)|37 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION - E (MCQs ASKED IN GUJET/BOARD EXAMS)|46 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-ELECTROCHEMISTRY-SECTION-B (Intext Questions and Answers)
- How would you determine the standard electrode potential of the system...
Text Solution
|
- Can you store copper sulphate solution in a zinc pot ?
Text Solution
|
- Consult the table of standard electrode potentials and suggets three s...
Text Solution
|
- Calculate the potential of hydrogen electrode in contact with a soluti...
Text Solution
|
- Calculate the emf of the cell in which the following reaction takes pl...
Text Solution
|
- The cell in which the following reactions occurs : 2Fe((aq))^(3+)+2l((...
Text Solution
|
- Why does the conductivity of a solution decrease with dilution ?
Text Solution
|
- Suggest a way to determine at Lamda(m)^(@) value of water.
Text Solution
|
- The molar conductivity of 0.025 mol L^(-1) methanoic acid is 46.1 S cm...
Text Solution
|
- If a current of 0.5 ampere flows through a metallic wire for 2 hours, ...
Text Solution
|
- Suggest a list of metals that are extracted electrolytically.
Text Solution
|
- Consider the reaction: Cr(2)O(2)^(2-)+14H^(+)+6e^(-) to 2Cr^(3+)+7H(...
Text Solution
|
- Write the chemistry of recharging the lead storage battery, highlighti...
Text Solution
|
- Suggest two materials other than hydrogen that can be used as fuels in...
Text Solution
|