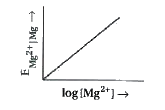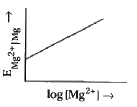A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
KUMAR PRAKASHAN|Exercise SECTION-D NCERT Exemplar Solution (MCQ.s More Than One Options)|10 VideosELECTROCHEMISTRY
KUMAR PRAKASHAN|Exercise SECTION-D NCERT Exemplar Solution (Short Answer Type Questions)|22 VideosELECTROCHEMISTRY
KUMAR PRAKASHAN|Exercise SECTION-C Textual Exercise|14 VideosCHEMISTRY IN EVERYDAY LIFE
KUMAR PRAKASHAN|Exercise SECTION - E (MCQs ASKED IN GUJCET/BOARD EXAM)|37 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION - E (MCQs ASKED IN GUJET/BOARD EXAMS)|46 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-ELECTROCHEMISTRY-SECTION-D NCERT Exemplar Solution (MCQ.s)
- Which cell will measure standard electrode potential of copper electro...
Text Solution
|
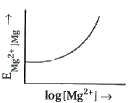
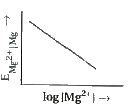
- Electrode potential for Mg electrode varies according to the equation ...
Text Solution
|
- Which of the following statement is correct ?
Text Solution
|
- The difference between the electrode potential of two electrodes when ...
Text Solution
|
- Which of the following statement is not correct about an inert electro...
Text Solution
|
- An electrochemical cell can behave like an electrolytic cell when .
Text Solution
|
- Which of the statements about solutions of electrolytes is not correct...
Text Solution
|
- Using the data given below find out the strongest reducing agent. E(...
Text Solution
|
- Us the data given in Q.B and find out which of the following is the st...
Text Solution
|
- Using the data given in Q.8 find out in which option the order of redu...
Text Solution
|
- Use the data given in q.8 and find out most stable ion is its reduced ...
Text Solution
|
- Us the data of Q.8 and find out ht emost stable oxidised species.
Text Solution
|
- The quantity of charge required to obtain one mole of aluminium from A...
Text Solution
|
- The cell constant of a conductivity cell
Text Solution
|
- Which charging the lead storage battery.
Text Solution
|
- Lamda(m(NH(4)OH))^(@) is equal to
Text Solution
|
- In the electrolysis of aqueous sodium chloride solution which of the h...
Text Solution
|