Text Solution
Verified by Experts
Topper's Solved these Questions
II PUC CHEMISTRY (ANNUAL EXAM QUESTION PAPER MARCH - 2014)
SUNSTAR PUBLICATION|Exercise PART - C|8 VideosII PUC CHEMISTRY ( ANNUAL EXAM QUESTION PAPER MARCH - 2020)
SUNSTAR PUBLICATION|Exercise PART-D|32 VideosII PUC CHEMISTRY (ANNUAL EXAM QUESTION PAPER MARCH - 2015)
SUNSTAR PUBLICATION|Exercise PART - D|26 Videos
Similar Questions
Explore conceptually related problems
SUNSTAR PUBLICATION-II PUC CHEMISTRY (ANNUAL EXAM QUESTION PAPER MARCH - 2014)-PART - D
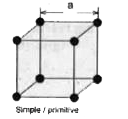
- Calculate the packing efficiency in a simple cubic lattice.
Text Solution
|
- What is Frenkel defect? Give an example.
Text Solution
|
- On dissolving 2.34g of non-electrolyte solute in 40g of benzene, the b...
Text Solution
|
- State Henry's law. Write its mathematical form.
Text Solution
|
- Draw a neat labeled diagram of Standard Hydrogen Electrode (SHE). Writ...
Text Solution
|
- b) Calculate Delta(r)G^(@) for the following reaction: Fe((aq))^(+2)...
Text Solution
|
- a) The rate of a particular reaction doubles when the temperature chan...
Text Solution
|
- b) Show that the half - life period of a first order reaction is indep...
Text Solution
|
- Write any two differences between physisorption and chemisorption.
Text Solution
|
- i) Mention the role of alum in the purification of drinking water. i...
Text Solution
|
- a) i) write the equations for the steps in SN1 mechanism of the conver...
Text Solution
|
- b) Complete the following equation: i) C(2)H(5)OH+SOCl(2)rarr ii)
Text Solution
|
- i) Explain the preparation of phenol from cumene. ii) Complete the r...
Text Solution
|
- Explain Williamson's ether synthesis.
Text Solution
|
- i) How do you convert benzoic acid to benzamide? Write the reaction. ...
Text Solution
|
- What happens when the carbonly compounds are treated with hydrazine? W...
Text Solution
|
- i) Explain Hoffmann bromamide degradation for the preparation of anili...
Text Solution
|
- b) What is Hinsberg's reagent? Between CH(3)NH(2) and C(6)H(5)NH(2) wh...
Text Solution
|
- i) Name the water insoluble component of starch. ii) Mention one wat...
Text Solution
|
- Write the structure of Maltose.
Text Solution
|