Text Solution
Verified by Experts
Topper's Solved these Questions
SUNSTAR PUBLICATION-II PUC CHEMISTRY ( ANNUAL EXAM QUESTION PAPER MARCH - 2020)-PART-D
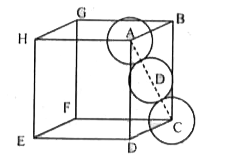
- Calculate the packing efficiency in Face Centred Cubic (FCC) structure...
Text Solution
|
- Calcium metal crystallises in a face centered cubic lattice with edge ...
Text Solution
|
- Vapour pressure of benzene is 200 mm of Hg. When 2 gram of a non-volat...
Text Solution
|
- What are azeotropes? Give an example for binary solutions showing mini...
Text Solution
|
- Calculate the e.m.f. of the cell in which the following reaction takes...
Text Solution
|
- State Kohlrausch law of independent migration of ions.
Text Solution
|
- Define limiting molar conductivity?
Text Solution
|
- Derive an intergrated rate for the first order reaction.
Text Solution
|
- According to collision theory, what are the two factors that lead to e...
Text Solution
|
- Write a note on Dialysis.
Text Solution
|
- What is the effect on AH and AS during the process of adsorption?
Text Solution
|
- Give an example for heterogeneous catalysis.
Text Solution
|
- Write equations for the steps in S(N)1 mechanism of conversion of tert...
Text Solution
|
- Complete the following reactions: CH3- CH = CH2 + HI to
Text Solution
|
- Complete the following reactions:
Text Solution
|
- Complete the following reactions: CH3CH2 Br underset("Aq. Ethanol")...
Text Solution
|
- Write the mechanism of acid catalysed dehydration of ethanol to ethene...
Text Solution
|
- How does anisole react with methyl chloride?
Text Solution
|
- How is benzoyl chloride converted into benzaldehyde. Write the equatio...
Text Solution
|
- Write a general equation for the formation of carboxylic acid from Gri...
Text Solution
|