A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
WB JEE PREVIOUS YEAR PAPER-QUESTION PAPER 2017-CHEMISTRY
- How many faradys are required to reduce 1 mole fo Cr(2)O(7) to Cr^(3-)...
Text Solution
|
- Equilibrium constant for the following reactions at 1200 K are given :...
Text Solution
|
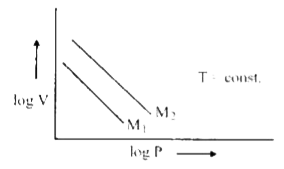
- For same mass of two different ideal gases of molecular weight M(1) an...
Text Solution
|
- Which of the following has the dimensions of ML^(0)T^(-2) ?
Text Solution
|
- If the given four electronic configurations i)n=4, l=1 ii)n=4, l=0...
Text Solution
|
- Which of the following sets of quantum numbers represnets the 19^(th) ...
Text Solution
|
- 0.126 g of an acid in needed to completely neutralize 20 ml 0.1 (NaH) ...
Text Solution
|
- In a flask, the weight ratio of CH(4)(g) and SO(2)(g) at 298 K and bar...
Text Solution
|
- C(6)H(5)F^(18) is a F^(18) radio-isotops labelled organic compound F^(...
Text Solution
|
- Dissolving NaCN in de-ionized water will result in a solution having
Text Solution
|
- Among Me(3)N, C(5) H(5)N and MeCN(Me= methyl group) the electronegativ...
Text Solution
|
- The shape of XeF(5)^(-) will be :
Text Solution
|
- The ground state, magnetic property of B(2) and C(2) molecules will be
Text Solution
|
- The number of unpaired electrons in [NiCl(4)]^(2-), Ni(CO)(4) and [Cu(...
Text Solution
|
- Which of the following atoms should have the highest 1^(st) electron a...
Text Solution
|
- PbCl(2) is insoluble in cold water Addition of HCI increases its solub...
Text Solution
|
- Of the following compounds which one is the strongest Bronsted acid i...
Text Solution
|
- The correct basiecity order of the following lanthanide ions is
Text Solution
|
- When BaCl(2) is added to an aqueous salt solution a white precipitate ...
Text Solution
|
- In the IUPAC system PhCH(2) CH(2)CO(2)H is named as
Text Solution
|