Text Solution
Verified by Experts
Topper's Solved these Questions
SOLVED PAPER II PUC JULY - 2016
OSWAAL PUBLICATION|Exercise PART -E|19 VideosSOLVED PAPER II PUC JULY - 2016
OSWAAL PUBLICATION|Exercise PART -C|12 VideosSOLVED PAPER II PUC APRIL-2016
OSWAAL PUBLICATION|Exercise PART-D |27 VideosSOLVED PAPER II PUC MARCH-2016
OSWAAL PUBLICATION|Exercise PART-D |24 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-SOLVED PAPER II PUC JULY - 2016-PART -D
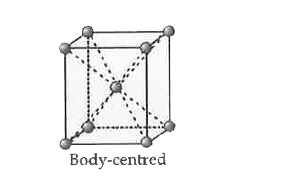
- Calculate packing efficiency in BCC lattice.
Text Solution
|
- Calculate the number of particles per unit cell in fcc.
Text Solution
|
- 300cm^(3) of an aqueous solution of a protein contains 2.12 g of the p...
Text Solution
|
- State Henry's law.
Text Solution
|
- i) State Henry's law. ii) Soda water bottles are sealed under high p...
Text Solution
|
- Find the value of AG^(@) at 25^(@)C for the following electrochemical ...
Text Solution
|
- Write the equations of anodic and cathodic reactions occur during rust...
Text Solution
|
- Derive an integrated rate equation for the rate constant of a zero ord...
Text Solution
|
- Write the energy distribution curve showing temperature dependence of ...
Text Solution
|
- Give two applications of adsorption.
Text Solution
|
- i) What is 'Tyndall effect'? ii) In the coagulation of negative sol,...
Text Solution
|
- i) What is 'Tyndall effect'? ii) In the coagulation of negative sol,...
Text Solution
|
- What is heterogeneous catalysis ? Give an example.
Text Solution
|