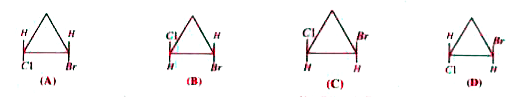A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ISOMERISM
AAKASH SERIES|Exercise PRACTICE SHEET (LEVEL-II) MORE THAN ONE CORRECT ANSWER TYPE QUESTIONS)|1 VideosISOMERISM
AAKASH SERIES|Exercise PRACTICE SHEET (LEVEL-II) LINKED COMPREHENSION TYPE QUESTIONS)|3 VideosISOMERISM
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE-III) STRAIGHT OBJECTIVE TYPE QUESTIONS)|20 VideosIONIC EQUILIBRIUM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL -II PRACTICE SHEET (ADVANCED) (Integer Type Questions))|8 VideosPERIODIC CLASSIFICATION
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (RECENT AIPMT/NEET QUESTIONS)|14 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ISOMERISM -PRACTICE SHEET (LEVEL-II) STRAIGHT OBJECTIVE TYPE QUESTIONS)
- The molecules below are
Text Solution
|
- Which pair of structures represents the same compound?
Text Solution
|
- In case of carbohydrates D, L indicates the
Text Solution
|
- Among the following which has L- configuration
Text Solution
|
- Which of the following can be used for resolving racemic carboxylic ac...
Text Solution
|
- A compound contains two dissimilar asymmetric carbon atoms. The number...
Text Solution
|
- Which of the following compounds do not exhibit steroisomerism
Text Solution
|
- Which of the following statements regarding I and II is right
Text Solution
|
- Which pair among the following is enantiomers
Text Solution
|
- Which of the following compound is (meso) compounds?
Text Solution
|
- Which of the following Fisher projection formulae represents same ster...
Text Solution
|
- Which of the following representation of the Fischer projection formul...
Text Solution
|
- Which of the following compounds have plane of symmetry?
Text Solution
|
- Among the following, the Newmann projection formula of meso-2, 3-butan...
Text Solution
|
- I) (A) and (B) are diastereomers II) (B) and (D) are en...
Text Solution
|
- Correct relationship between the two structures given below is
Text Solution
|
- 2,3-Butanediol has the 2R, 3R configuration Identify the correct state...
Text Solution
|
- Which statement is true for meso compound?
Text Solution
|
- Correct statement about
Text Solution
|
- How many of the following have chiral centres
Text Solution
|