A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ISOMERISM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE ( PRACTICE SHEET (ADVANCED) LINKED COMPREHENSION TYPE QUESTIONS)|8 VideosISOMERISM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE ( PRACTICE SHEET (ADVANCED) MATRIX MATCHING TYPE QUESTIONS)|1 VideosISOMERISM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL-II LECTURE SHEET (ADVANCED) INTEGER TYPE QUESTIONS)|11 VideosIONIC EQUILIBRIUM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL -II PRACTICE SHEET (ADVANCED) (Integer Type Questions))|8 VideosPERIODIC CLASSIFICATION
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (RECENT AIPMT/NEET QUESTIONS)|14 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ISOMERISM -ADDITIONAL PRACTICE EXERCISE ( PRACTICE SHEET (ADVANCED) MORE THAN ONE CORRECT ANSWER TYPE QUESTIONS)
- Which compound show tautomerism:
Text Solution
|
- Which of the following compounds exhibits Tautomerism
Text Solution
|
- Which of the following niolecule does not contain chiral centre (s)
Text Solution
|
- For which of the following pairs of compounds are the CORRECT notation...
Text Solution
|
- Configuration structure of which compound (s) correctly predict the po...
Text Solution
|
- Which of the following statement is right
Text Solution
|
- Which of the following is not the correct relationship
Text Solution
|
- Which of the following alkanes give only one mono-chloro derivative
Text Solution
|
- The correct order of % enol content.
Text Solution
|
- In Which of the following pairs I is more stable than II ?
Text Solution
|
- Determine which of the following compounds is a trans isomer.
Text Solution
|
- Which of the following statements regarding the Fischer projection for...
Text Solution
|
- is optically inactive due to the presence of
Text Solution
|
- Which of the following molecules is achiral?
Text Solution
|
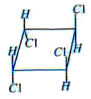 is optically inactive due to the presence of
is optically inactive due to the presence of