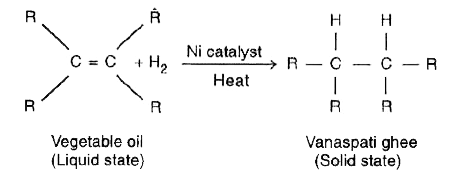Text Solution
Verified by Experts
Topper's Solved these Questions
CARBON AND ITS COMPOUNDS
OSWAAL PUBLICATION|Exercise Topic II- Carbon Compounds , Soap and Detergents (Long Answer Type Questions)|15 VideosCARBON AND ITS COMPOUNDS
OSWAAL PUBLICATION|Exercise NCERT Corner (Intext Questions)|13 VideosCARBON AND ITS COMPOUNDS
OSWAAL PUBLICATION|Exercise Topic II- Carbon Compounds , Soap and Detergents (Short Answer Type Question-I)|11 VideosACIDS,BASES AND SALTS
OSWAAL PUBLICATION|Exercise NCERT CORNER TEXTBOOK EXERCISES|15 VideosCHEMICAL REACTIONS AND EQUATIONS
OSWAAL PUBLICATION|Exercise NCERT CORNER (Textbook Exercises)|20 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-CARBON AND ITS COMPOUNDS-Topic II- Carbon Compounds , Soap and Detergents (Short Answer Type Question-II)
- An organic compound 'P' is a constituent of wine. 'P' on reacting with...
Text Solution
|
- Write chemical equation of the reaction of ethanoic acid with the foll...
Text Solution
|
- On dropping a small piece of sodium in a test-tube containing carbon c...
Text Solution
|
- When we take 1 ml ethanol and 1 ml ethanoic acid along with a few drop...
Text Solution
|
- With the help of an example, explain the process of hydrogenation. Men...
Text Solution
|
- What is the difference between the molecules of soaps and detergents, ...
Text Solution
|
- Write the name and structural formula of the compound formed when etha...
Text Solution
|
- What are esters ? How they are prepared ? List two uses of esters.
Text Solution
|
- Draw the electron dot structure of ethyne. A mixture of ethyne and ony...
Text Solution
|
- Write the chemical equations for the following chemical reactions and ...
Text Solution
|
- Name the oxidising agents used for the conversion of ethanol to ethano...
Text Solution
|
- Complete the following equations : (i) CH(3)COOC(2)H(5)overset(NaOH)...
Text Solution
|
- How are the following products obtained from ethanol ? (i) Ethyl et...
Text Solution
|
- An organic compound of molecular formula C(2)H(4) on reduction gives a...
Text Solution
|
- Two compounds 'A' and 'B' have the molecular formula C(3)H(8) and C(3)...
Text Solution
|
- (i) Give a kherical test to distinguish between saturated and unsatura...
Text Solution
|
- An organic compound 'A' of molecular formula C(2)H(6)O on oxidation wi...
Text Solution
|
- (i) Write chemical name and formula of Vinegar. (ii) Describe with a...
Text Solution
|
- (i) Write the chemical names of CH(3)COCH(3),C(2)H(5)OH. (ii) What h...
Text Solution
|
- Complete the following reactions : (i) CH(3)CH(2)OHoverset"Alkaline"...
Text Solution
|