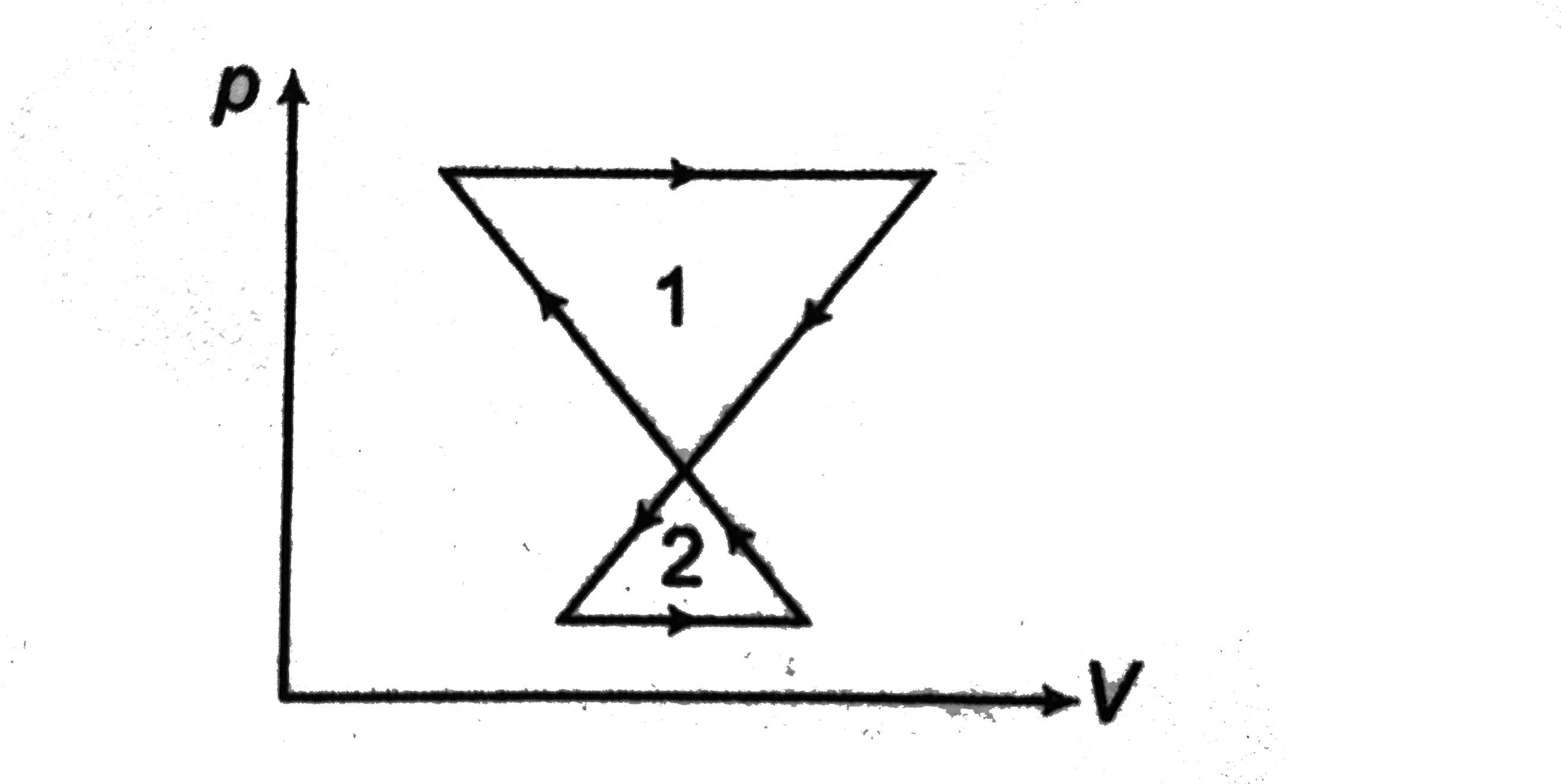
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
LAWS OF THERMODYNAMICS
DC PANDEY ENGLISH|Exercise Exercise 21.3|7 VideosLAWS OF THERMODYNAMICS
DC PANDEY ENGLISH|Exercise Exercise 21.4|8 VideosLAWS OF THERMODYNAMICS
DC PANDEY ENGLISH|Exercise Exercise 21.1|1 VideosLAWS OF MOTION
DC PANDEY ENGLISH|Exercise Medical entrances gallery|39 VideosMAGNETIC EFFECT OF CURRENT AND MAGNETISM
DC PANDEY ENGLISH|Exercise Integer type Questions|10 Videos
Similar Questions
Explore conceptually related problems
DC PANDEY ENGLISH-LAWS OF THERMODYNAMICS-Exercise 21.2
- A gas in a cylinder is held at a constant pressure of 1.7xx10^5 Pa and...
Text Solution
|
- A thermodynamic system undergoes a cyclic process as shown in figure. ...
Text Solution
|
- How many moles of helium at temperature 300K and 1.00 atm pressure are...
Text Solution
|
- Temperature of four moles of a monoatomic gas is increased by 300K in ...
Text Solution
|
- Find work done by the gas in the process AB shown in the following fig...
Text Solution
|
- Temperature of two moles of an ideal gas is increased by 300K in a pro...
Text Solution
|
- Pressure and volume of a gas changes from (p0V0) to (p0/4, 2V0) in a p...
Text Solution
|