Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
LAWS OF THERMODYNAMICS
DC PANDEY ENGLISH|Exercise Level 2 Single Correct|27 VideosLAWS OF THERMODYNAMICS
DC PANDEY ENGLISH|Exercise Level 2 More Than One Correct|6 VideosLAWS OF THERMODYNAMICS
DC PANDEY ENGLISH|Exercise Level 1 Objective Questions|1 VideosLAWS OF MOTION
DC PANDEY ENGLISH|Exercise Medical entrances gallery|39 VideosMAGNETIC EFFECT OF CURRENT AND MAGNETISM
DC PANDEY ENGLISH|Exercise Integer type Questions|10 Videos
DC PANDEY ENGLISH-LAWS OF THERMODYNAMICS-Level 1 Subjective
- A thermodynamic system undergoes a cyclic process as shown in figure. ...
Text Solution
|
- A gas undergoes the cycle shown in figure. The cycle is repeated 100 t...
Text Solution
|
- One mole of an ideal monoatomic gas is initially at 300K. Find the fin...
Text Solution
|
- A closed vessel 10L in volume contains a diatomic gas under a pressure...
Text Solution
|
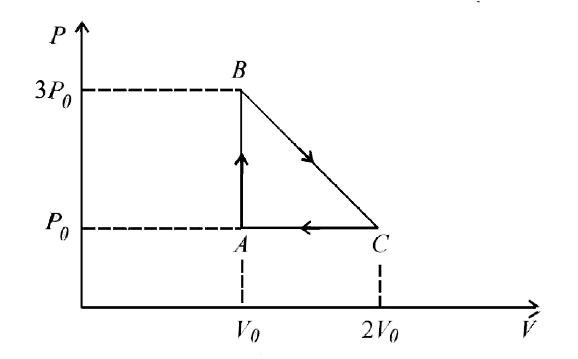
- One mole of an ideal monatomic gas is taken round the cyclic process A...
Text Solution
|
- A diatomic ideal gas is heated at constant volume until its pressure b...
Text Solution
|
- Two moles of a certain gas at a temperature T0=300K were cooled isocho...
Text Solution
|
- Five moles of an ideal monoatomic gas with an initial temperature of 1...
Text Solution
|
- Find the change in the internal energy of 2 kg of water as it heated f...
Text Solution
|
- Calculate the increase in the internal energy of 10 g of water when it...
Text Solution
|
- One gram of water (1 cm^3) becomes 1671 cm^3 of steam when boiled at a...
Text Solution
|
- A gas in a cyclinder is held at a constant pressure of 2.30xx10^5 Pa a...
Text Solution
|
- p-V diagram of an ideal gas for a process ABC is as shown in the figur...
Text Solution
|
- In the given graph, an ideal gas changes its state from A to C by two ...
Text Solution
|
- When a gas expands along AB, it does 500J of work and absorbs 250 J of...
Text Solution
|
- A 1.0 kg bar of copper is heated at atmospheric pressure (1.01xx10^5N/...
Text Solution
|
- One mole of an ideal monoatomic gas occupies a volume of 1.0xx10^-2m^3...
Text Solution
|
- A bullet of mass 10g travelling horizontally at 200m//s strikes and em...
Text Solution
|
- An ideal gas is carried through a thermodynamic cycle consisting of tw...
Text Solution
|
- An ideal gas is enclosed in a cyclinder with a movable piston on top. ...
Text Solution
|