Text Solution
Verified by Experts
Topper's Solved these Questions
SOLVED PAPER II PUC MARCH-2016
OSWAAL PUBLICATION|Exercise PART-D |24 VideosSOLVED PAPER II PUC MARCH-2016
OSWAAL PUBLICATION|Exercise PART-B |9 VideosSOLVED PAPER II PUC JULY - 2016
OSWAAL PUBLICATION|Exercise PART -E|19 VideosSOLVED PAPER II PUC TOPPER'S ANSWER MARCH-2015
OSWAAL PUBLICATION|Exercise PART -D|30 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-SOLVED PAPER II PUC MARCH-2016 -PART-C
- For the manufacture of Ammonia by Haber's process, write the equation ...
Text Solution
|
- How is pure alumina obtained from bauxite by leaching process.
Text Solution
|
- How is chlorine prepared in the laboratory using KMNo(4) ?
Text Solution
|
- Inter halogen compounds are more reactive than halogens . Why ?
Text Solution
|
- Among the following which one is more acidic ? Give reason. H(2)O,H(...
Text Solution
|
- Complete the following equation. H(2)SO(4)+So(3)rarr?
Text Solution
|
- With the help of Valence Bond theory account for hybridisation, geomet...
Text Solution
|
- Explain the preparation of potassium permanganate from MnO(2) Write th...
Text Solution
|
- Give reason : SC^(3+) ions are colourless whereas V^(3+) ions are ...
Text Solution
|
- (a) What are interstitial compounds ? Write any one of their character...
Text Solution
|
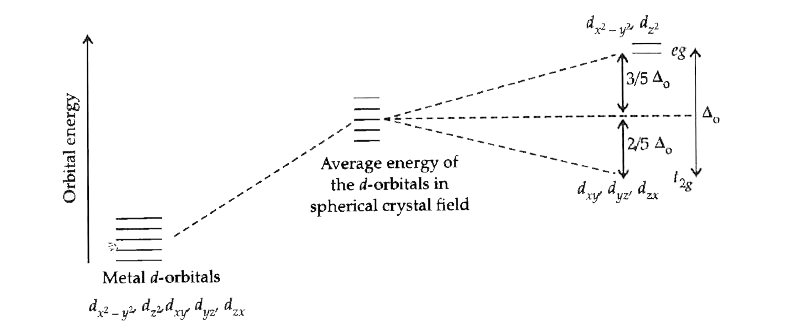
- Explain the Crystal field splitting is an octahedral field.
Text Solution
|