Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-SOLVED PAPER II PUC MARCH-2016 -PART-D
- Calculate the packing efficiency in a simple cubic lattice.
Text Solution
|
- An element crystallizes in fcc lattice. If the edge length of the unit...
Text Solution
|
- Give reason for the following. Brownian movement of colloidal partic...
Text Solution
|
- What is peptization ? Give an example.
Text Solution
|
- Derive an integrated rate equation for the rate constant of a first-or...
Text Solution
|
- Draw the graph for [R] versus time (t) for a zero order reaction. Give...
Text Solution
|
- Write the mechanism of acid catalysed dehydration of ethanol to ethene...
Text Solution
|
- What is the effect of the following groups on the acidity of phenol ? ...
Text Solution
|
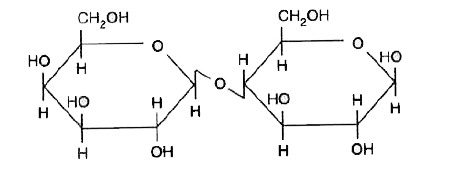
- (a) Write the Haworth's structure for lactose. (b) Give an example ...
Text Solution
|
- Explain S(N) - 1 reaction mechanism.
Text Solution
|
- Explain Fittig reaction with equation.
Text Solution
|
- What is cross aldol condensation. Give an example.
Text Solution
|
- Explain decarboxylation reaction with an example.
Text Solution
|
- What is the major product of the following reaction ?
Text Solution
|
- What are condensation polymers? Given an example.
Text Solution
|
- Write the following: (i) IUPAC name for the monomer of natural rubb...
Text Solution
|
- Give an example for a co-polymer.
Text Solution
|
- Explain Hoffmann bromamide degradation for the preparation of methanam...
Text Solution
|
- Complete the following reactions : (i) (ii) C(6)H(5)NH(2) +NaNO(2...
Text Solution
|
- Give reason: Ammonia is more basic than aniline.
Text Solution
|