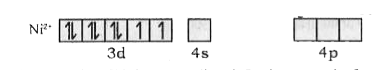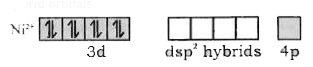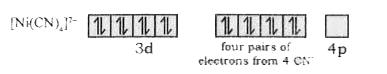Text Solution
Verified by Experts
Topper's Solved these Questions
II PUC CHEMISTRY (SUPPLEMENTARY EXAM QUESTION PAPER JULY - 2014)
SUNSTAR PUBLICATION|Exercise PART - D|22 VideosII PUC CHEMISTRY (SUPPLEMENTARY EXAM QUESTION PAPER JULY - 2014)
SUNSTAR PUBLICATION|Exercise PART - B|8 VideosII PUC CHEMISTRY (P.U. BOARD LATEST MODEL QUESTION PAPER - 3)
SUNSTAR PUBLICATION|Exercise PART - D|30 VideosII PUC CHEMISTRY (SUPPLEMENTARY EXAM QUESTION PAPER JULY - 2016)
SUNSTAR PUBLICATION|Exercise PART - D|25 Videos
Similar Questions
Explore conceptually related problems
SUNSTAR PUBLICATION-II PUC CHEMISTRY (SUPPLEMENTARY EXAM QUESTION PAPER JULY - 2014)-PART - C
- Describe the three steps involved in the leaching of bauxite to get p...
Text Solution
|
- How is ammonia manufactured by Haber's process?
Text Solution
|
- Describe the preparation of Ozonised oxygen with an equation. Name the...
Text Solution
|
- Complete the following equatious: i) 2Al+3Cl(2)rarr ii) H(2)S+Cl(2...
Text Solution
|
- How is potassium dichromate prepared from chromite are ?
Text Solution
|
- Why do transition elements from complex compounds? Name the elements w...
Text Solution
|
- State any three postulates of Werner theory of co-ordination compounds...
Text Solution
|
- Using VBT, explainthe geometry and magnetic property of [Ni(CN)(4)]^(-...
Text Solution
|