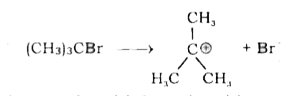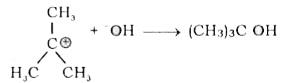Text Solution
Verified by Experts
Topper's Solved these Questions
II PUC CHEMISTRY (SUPPLEMENTARY EXAM QUESTION PAPER JULY - 2014)
SUNSTAR PUBLICATION|Exercise PART - C|8 VideosII PUC CHEMISTRY (P.U. BOARD LATEST MODEL QUESTION PAPER - 3)
SUNSTAR PUBLICATION|Exercise PART - D|30 VideosII PUC CHEMISTRY (SUPPLEMENTARY EXAM QUESTION PAPER JULY - 2016)
SUNSTAR PUBLICATION|Exercise PART - D|25 Videos
Similar Questions
Explore conceptually related problems
SUNSTAR PUBLICATION-II PUC CHEMISTRY (SUPPLEMENTARY EXAM QUESTION PAPER JULY - 2014)-PART - D
- The vapour pressure of pure benzene at certain temperature is 0.850 ba...
Text Solution
|
- What happens to the solubility of a gas in a liquid on increasing temp...
Text Solution
|
- Describe the construction and working of standard hydrogen electrode.
Text Solution
|
- State Faraday's second law of electrolysis.
Text Solution
|
- a) The rate of a particular reaction doubles when the temperature chan...
Text Solution
|
- Write any two differences between order and molecularity of a reaction...
Text Solution
|
- What is coagulation of a sol? Name two methods by which a lyophobic so...
Text Solution
|
- What is homogenous catalysis? Give an example.
Text Solution
|
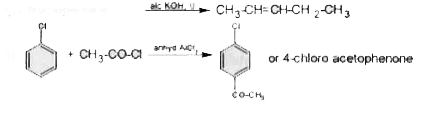
- Mention the major product formed in the following reaction: i) ii...
Text Solution
|
- Write equations for the steps in SN^1 mechanism of the conversion of t...
Text Solution
|
- What is the effect of i) Electron with drawing group on acidity of p...
Text Solution
|
- Write the general equation of Williamson's ether synthesis
Text Solution
|
- Write equation for: i) The reaction between Carboxylic acid and PCl(...
Text Solution
|
- Explain aldol condensation with an example.
Text Solution
|
- i) CH(3)-CO-NH(2)overset(Br(2)//NaOH)rarr ii) Poverset(NaNO(2), HCl)...
Text Solution
|
- Explain carbylamine reaction.
Text Solution
|
- i) What is denaturation of protein? ii) Give an example of acidic am...
Text Solution
|
- Write the Haworth's structure of -D(+) Glucose.
Text Solution
|
- Name the monomers used for getting following polymers: i) PVC ii) Ba...
Text Solution
|
- What is vulcanisation of rubber?
Text Solution
|