Text Solution
Verified by Experts
Topper's Solved these Questions
II PUC CHEMISTRY (ANNUAL EXAM QUESTION PAPER MARCH - 2016)
SUNSTAR PUBLICATION|Exercise PART - D|26 VideosII PUC CHEMISTRY (ANNUAL EXAM QUESTION PAPER MARCH - 2016)
SUNSTAR PUBLICATION|Exercise PART - B|8 VideosII PUC CHEMISTRY (ANNUAL EXAM QUESTION PAPER MARCH - 2015)
SUNSTAR PUBLICATION|Exercise PART - D|26 VideosII PUC CHEMISTRY (P.U. BOARD LATEST MODEL QUESTION PAPER - 1)
SUNSTAR PUBLICATION|Exercise PART - D|25 Videos
Similar Questions
Explore conceptually related problems
SUNSTAR PUBLICATION-II PUC CHEMISTRY (ANNUAL EXAM QUESTION PAPER MARCH - 2016)-PART - C
- In the extraction of Aluminimum by electrolysis. i. Give the composi...
Text Solution
|
- Write the balanced Chemical equation with condition involved in manufa...
Text Solution
|
- Complete the following chemical equations. i. PbS+4O(3)rarr PbSO(4)+...
Text Solution
|
- How is chlorine prepared using KMnO4 ?
Text Solution
|
- Why is I2 less reactive that ICl?
Text Solution
|
- Calculate the spin only magnetic moment of Fe^(2+)
Text Solution
|
- Why Sc^(3+) salts are colourless whereas Cr^(3+) salts are coloured.
Text Solution
|
- Write the balanced equation in the manufacture of K2Cr2O7 from chromit...
Text Solution
|
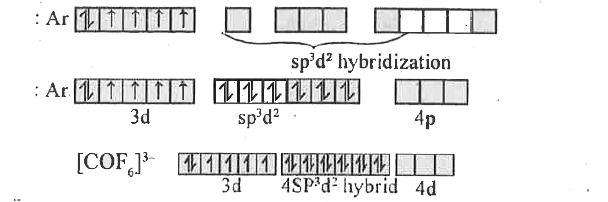
- On the basis of VBT explain the hybridization, geometrical shape and m...
Text Solution
|
- Write any two postulates of Werner's theory of co-ordination compounds...
Text Solution
|
- Write the IUPAC name of [Pt(NH(3))(2)(H(2)O)Cl(2))
Text Solution
|