Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MAXIMUM PUBLICATION-STRUCTURE OF ATOM-EXAMPLE
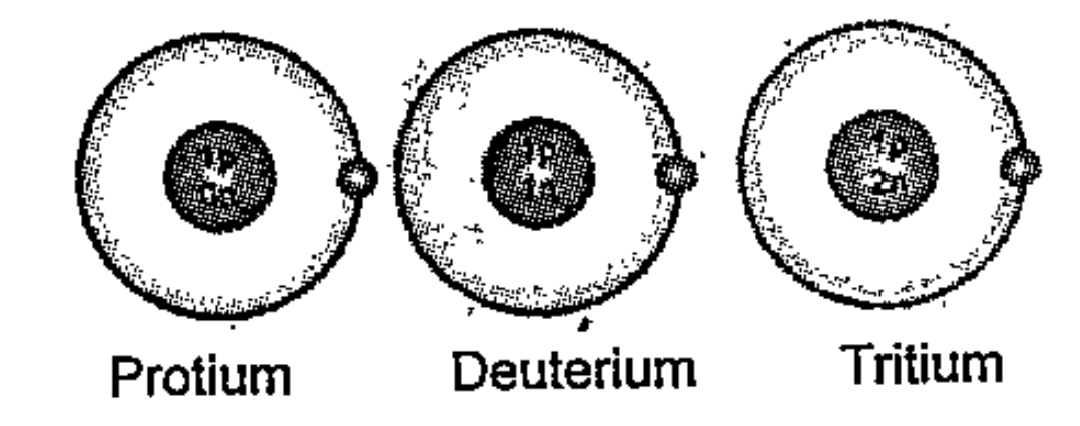
- Complete the table providing details related to these atoms.
Text Solution
|
- Among these atoms which is the particle that is differs in numbers?
Text Solution
|
- Which inferences can you arrive at when you examine the atomic number ...
Text Solution
|
- What is meant by Isotopes?
Text Solution
|
- Which isotope of Hydrogen is used in atomic reactors?
Text Solution
|
- Write the isotopes of carbon?
Text Solution
|
- Which are the isotopes is used as tracers for identifying the nutrient...
Text Solution
|
- Which isotope of Uranium is used in atomic reactors as fuel?
Text Solution
|
- what is meant by isobar?
Text Solution
|
- Symbols of certain isotopes are given in table ,Complete the table wri...
Text Solution
|
- Name of some scientists and their contributions are given in in the fo...
Text Solution
|
- Atomic number of an atom Z=17, Mass number 35. Find the number of pr...
Text Solution
|
- Atomic number of an atom Z=17, Mass number 35. Write the electronic...
Text Solution
|
- Atomic number of an atom Z=17, Mass number 35. Draw the Bohr model ...
Text Solution
|
- The mass number of an atom is 31. The M shell of this atom contains 5 ...
Text Solution
|
- The mass number of an atom is 31. The M shell of this atom contains 5 ...
Text Solution
|
- The mass number of an atom is 31. The M shell of this atom contains 5 ...
Text Solution
|
- The mass number of an atom is 31. The M shell of this atom contains 5 ...
Text Solution
|
- Bohr models of atoms A, B, C, are given (Symbols are not real). Wr...
Text Solution
|
- Bohr models of atoms A, B, C, are given (Symbols are not real). Am...
Text Solution
|