Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MAXIMUM PUBLICATION-STRUCTURE OF ATOM-EXAMPLE
- The mass number of an atom is 31. The M shell of this atom contains 5 ...
Text Solution
|
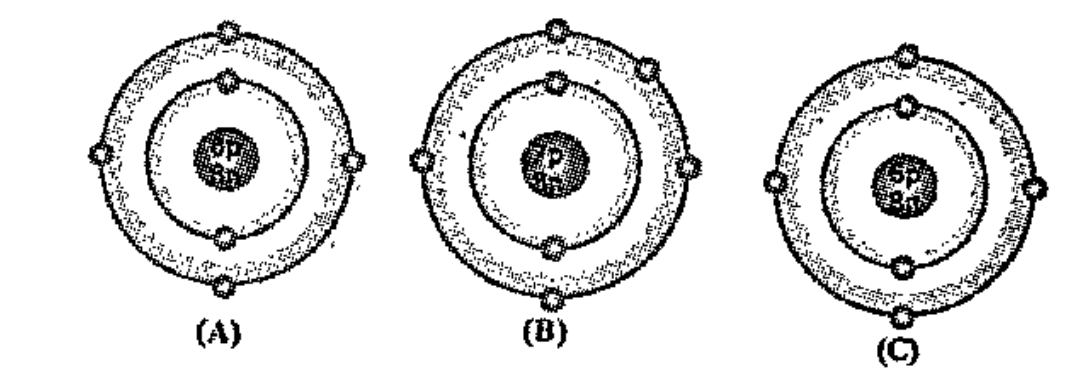
- Bohr models of atoms A, B, C, are given (Symbols are not real). Wr...
Text Solution
|
- Bohr models of atoms A, B, C, are given (Symbols are not real). Am...
Text Solution
|
- Symbols (not real symbols) of some atoms are given. 8^17P , 18^4...
Text Solution
|
- Symbols (not real symbols) of some atoms are given. 8^17P , 18^4...
Text Solution
|
- Symbols (not real symbols) of some atoms are given. 8^17P , 18^4...
Text Solution
|
- Write the atomic number of an atom is 18 and mass number is 40 Find...
Text Solution
|
- Write the atomic number of an atom is 18 and mass number is 40 Writ...
Text Solution
|
- Write the atomic number of an atom is 18 and mass number is 40 Draw...
Text Solution
|
- The 3rd shell ie. M shell of an atom contains 3 electrons. The mass nu...
Text Solution
|
- The 3rd shell ie. M shell of an atom contains 3 electrons. The mass nu...
Text Solution
|
- The 3rd shell ie. M shell of an atom contains 3 electrons. The mass nu...
Text Solution
|
- The 3rd shell ie. M shell of an atom contains 3 electrons. The mass nu...
Text Solution
|
- Write the uses of Carbon -14
Text Solution
|
- Write the uses of Phosphorous - 31
Text Solution
|
- Write the uses of Iodine - 131 and cobalt 60
Text Solution
|
- Write the uses of Uranium - 235
Text Solution
|
- Write the electronic configuration and draw the Bohr model? 17^35Cl
Text Solution
|
- Write the electronic configuration and draw the Bohr model? 11^23Na
Text Solution
|
- 6^12C , 8^40Ar , 6^14C , 20^40Ca Find the isotopes and isobars pair...
Text Solution
|