Text Solution
Verified by Experts
Topper's Solved these Questions
INTERNAL ENERGY
ICSE|Exercise SELECTED PROBLEMS (FROM HEAT ENGINES)|21 VideosINTERNAL ENERGY
ICSE|Exercise SELECTED PROBLEMS (FROM ISOTHERMAL AND ADIABATIC PROCESSES )|10 VideosGRAVITATION
ICSE|Exercise FROM THE HUBBLE TELESCOP|2 VideosMOTION IN FLUIDS
ICSE|Exercise SELECTED PROBLEMS (FROM POISEUILLE.S FORMULA) |19 Videos
Similar Questions
Explore conceptually related problems
ICSE-INTERNAL ENERGY -SELECTED PROBLEMS (FROM WORK DONE AND INDICATOR DIAGRAM )
- In changing the state of a gas adiabatically from an equilibrium state...
Text Solution
|
- When a gas is heated its volume increases from 100 cc to 500cc, at a c...
Text Solution
|
- For a particular gas gamma = (5)/(3)and is heated at constant pressure...
Text Solution
|
- A certain quantity of air is adiabatically compressed so that its pres...
Text Solution
|
- A gas occupying a volume of 10^(-2) m ^(3). at a pressure of 5 atmosp...
Text Solution
|
- Calculate the minimum work that must be done to compress 1.0 gm of hyd...
Text Solution
|
- An ideal gas of volume 1 litre and at a pressure 8 atm undergoes an ad...
Text Solution
|
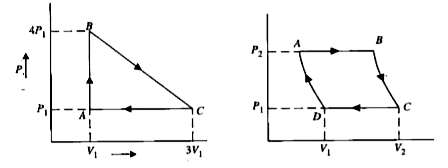
- The PV diagram for cyclic process is a triangle ABC see Fig. drawn in ...
Text Solution
|
- Two moles of helium gas undergo a cyclic process as shown in Fig. 19.2...
Text Solution
|
- An ideal gas is taken around the cycle ABCA as shown in P-V diagram. T...
Text Solution
|
- An ideal gas undergoes a thermodynamic process as shown in Fig. The pr...
Text Solution
|
- A gas expands from A to C along three different paths as shown in Fig....
Text Solution
|
- consider the cyclic process ABCA on a sample of 2.0 mol of an ideal ga...
Text Solution
|