Topper's Solved these Questions
ELECTROLYSIS
ICSE|Exercise WORK SHEET -3 (Fill in the blanks )|10 VideosELECTROLYSIS
ICSE|Exercise WORK SHEET -3 (Give reasons with regard to electroplating : )|4 VideosELECTROLYSIS
ICSE|Exercise WORK SHEET -1|17 VideosCHEMISTRY-2015
ICSE|Exercise SECTION-II (40 Marks) Attempt any four questions from this Section.|49 VideosMETALLURGY
ICSE|Exercise UNIT TEST PAPER 6 - Metallurgy (Select the correct answer from the list A,B,C & given in each statement.) |5 Videos
Similar Questions
Explore conceptually related problems
ICSE-ELECTROLYSIS-WORK SHEET -2
- Ion present ......... in the electrochemical series gets discharged at...
Text Solution
|
- Electrodes that do not take part in electrolytic, reactions are known ...
Text Solution
|
- Electrodes made of copper, nickel, silver are known as ..................
Text Solution
|
- Electrolytic cell used for electrolysis of water is known as ............
Text Solution
|
- .......... gas is liberated at anode during electrolysis of water.
Text Solution
|
- The ions which migrate towards an electrode but remains unaffected are...
Text Solution
|
- The ions which get deposited have ............ reduction potential.'
Text Solution
|
- Electrode at which reduction takes place is known as ........
Text Solution
|
- The frequency of alternating current is .........
Text Solution
|
- ........... electrodes are used during electrolysis of acidulated wate...
Text Solution
|
- A graphite anode is preferred to other inert electrodes during electro...
Text Solution
|
- During electrolysis of molten lead bromide a redox reaction is said to...
Text Solution
|
- Aluminium is extracted from its oxide by electrolytic reduction and no...
Text Solution
|
- Why is lead bromide maintained in molten state ?
Text Solution
|
- Why is the electrolytic cell made of silica ?
Text Solution
|
- Name the ions present in the electrolyte.
Text Solution
|
- What would you observe at the (a) cathode (b) anode ?
Text Solution
|
- Summarize the electrode reactions.
Text Solution
|
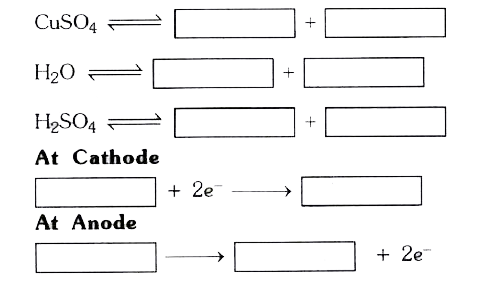
- Complete the following table with respect to electrolysis of acidulate...
Text Solution
|