Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SOLIDS-Exercise 21(B)
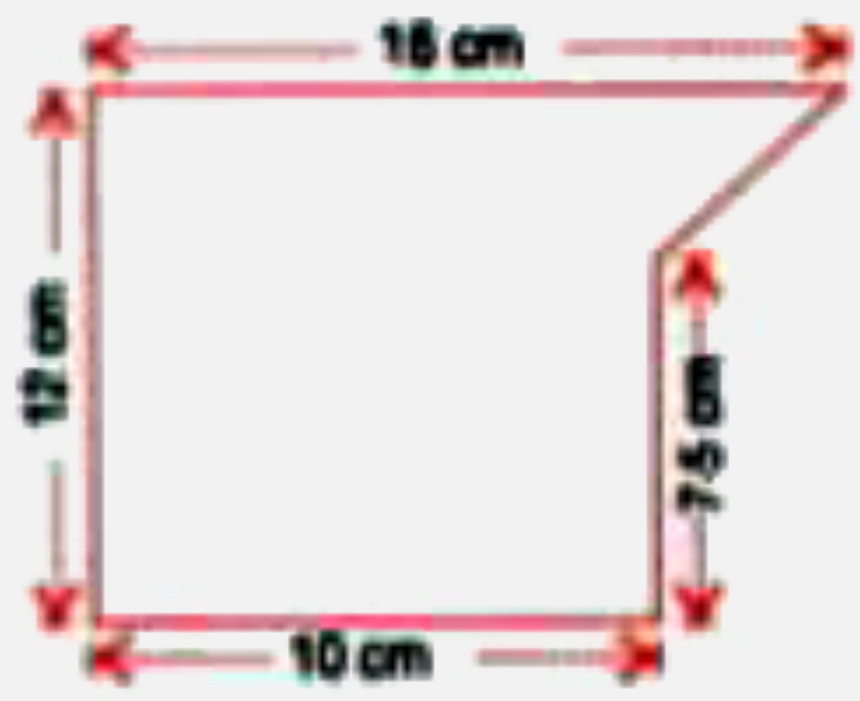
- The following figure shows a solid of uniform cross-section. Find the ...
Text Solution
|
- A swimming pool is 40m long and 15m wide. Its shallow and deep ends ar...
Text Solution
|
- The cross-section of a tunnel perpendicular to its length is a trapezi...
Text Solution
|
- The cross-section of a tunnel perpendicular to its length is a trapezi...
Text Solution
|
- Water is discharged from a pipe of cross-section area 3.2cm^(2) at the...
Text Solution
|
- Water is discharged from a pipe of cross-section area 3.2cm^(2) at the...
Text Solution
|
- A hose-pipe of cross-section area 2cm^(2) delivers 1500 litres of wate...
Text Solution
|
- The cross-section of a piece of metal 4m in length is shown below. Cal...
Text Solution
|
- The cross-section of a piece of metal 4m in length is shown below. Cal...
Text Solution
|
- A rectangular water-tank measuring 80cm xx 60cm xx 60cm is filled from...
Text Solution
|
- A rectangular card-board sheet has length 32cm and breadth 26cm. Squar...
Text Solution
|
- A swimming pool is 18m long and 8m wide. Its deep and shallow ends are...
Text Solution
|
- The following figure shows a closed victory-stand whose dimensions are...
Text Solution
|