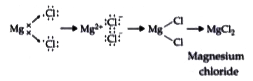Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE PAPER 2017-SECTION-II
- Identify the salts Q from the observations given below: When dilute...
Text Solution
|
- Draw an electron dot diagram to show the formation of each of the foll...
Text Solution
|
- Draw an electron dot diagram to show the formation of each of the foll...
Text Solution
|
- State the observations at the anode and at the cathode during the elec...
Text Solution
|
- State the observations at the anode and at the cathode during the elec...
Text Solution
|
- Select the ion in each case , that would get slectively discharged fro...
Text Solution
|
- Select the ion in each case, that would get selectively discharged fro...
Text Solution
|
- Certain blank spaces are left in the following table and these are lab...
Text Solution
|
- Write balanced chemical equations to show: (i) The oxidizing action...
Text Solution
|
- Write balanced chemical equations to show: The behaviour of H(2)SO(...
Text Solution
|
- Write balanced chemical equations to show: The dehydrating propert...
Text Solution
|
- Write balanced chemical equations to show how SO3 is converted to Sulp...
Text Solution
|
- Propane burns in air according to the following equation : C3H8 +5O2...
Text Solution
|
- The mass of 11.2 litre of a certain gas at S.T.P. is 24 g. Find the gr...
Text Solution
|
- A gas cylinder can hold 1 kg of hydrogen at room temperature and press...
Text Solution
|
- A gas cylinder can hold 1 kg of hydrogen at room temperature and press...
Text Solution
|
- A gas cylinder can hold 1 kg of hydrogen at room temperature and press...
Text Solution
|
- A gas cylinder can hold 1 kg of hydrogen at room temperature and press...
Text Solution
|
- Write a balanced chemical equation for the preparation of the followin...
Text Solution
|
- Write a balanced chemical equation for the preparation of the followin...
Text Solution
|