Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE PAPER 2018-SECTION-II
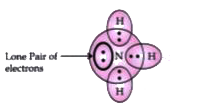
- What do you understand by a lone pair of electrons ?
Text Solution
|
- Draw the electron dot diagram of Hydronium ion. (H = 1, O = 8)
Text Solution
|
- In Period 3 of the Periodic Table, element B is placed to the left of...
Text Solution
|
- In Period 3 of the Periodic Table, element B is placed to the left of ...
Text Solution
|
- In Period 3 of the Periodic Table, element B is placed to the left of...
Text Solution
|
- Complete the following table which refers to the conversion of ions to...
Text Solution
|
- Write the balanced chemical equation to prepare ammonia gas in the lab...
Text Solution
|
- State why concentrated sulphuric acid is not used for drying ammonia g...
Text Solution
|
- Why is ammonia gas not collected over water?
Text Solution
|
- Name the acid used for the preparation of hydrogen chloride gas in the...
Text Solution
|
- Write the balanced chemical equation for the laboratory preparation of...
Text Solution
|
- For the preparation of hydrochloric acid in the laboratory : Why is...
Text Solution
|
- For the preparation of hydrochloric acid in the laboratory : What a...
Text Solution
|
- For the electro-refining of copper What is the cathode made-up of ?
Text Solution
|
- For the electro-refining of copper Write the reaction that takes pl...
Text Solution
|
- The percentage composition of a gas is : Nitrogen 82.35%, Hydrogen 17....
Text Solution
|
- Aluminium carbide reacts with water according to the following equatio...
Text Solution
|
- Aluminium carbide reacts with water according to the following equatio...
Text Solution
|
- If 150 cc of gas A contains X molecules, how many molecules of gas B w...
Text Solution
|
- If 150 cc of gas A contains X molecules, how many molecules of gas B w...
Text Solution
|