Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE PAPER 2020-SECTION-II (40 Marks) Attempt any four questions from this Section
- Calculate the percentage of : Sodium in sodium aluminium fluoride...
Text Solution
|
- Calculate the percentage of : Aluminium in sodium aluminium fluori...
Text Solution
|
- State the volume occupied by 40 gm of methane at STP, if its vapour de...
Text Solution
|
- Calculate the number of moles present in 160 gm of NaOH. [Atomic Mass ...
Text Solution
|
- Identify the salts P, Q, R from the observation Salt P has light blu...
Text Solution
|
- Identify the salt Q from the observation Salt Q is white in colour....
Text Solution
|
- Identify the salt R from the observation Salt R is black in colour. ...
Text Solution
|
- Identify the substance underlined The ul"electrode" that increases...
Text Solution
|
- Identify the substance underlined The ul"acid" that is a dehydrati...
Text Solution
|
- Identify the substance italicised : The catalyst used to oxidise ammon...
Text Solution
|
- Copy and complete the following paragraph using the options given in b...
Text Solution
|
- Write balanced chemical equations, for the preparation of the given sa...
Text Solution
|
- Write balanced chemical equations, for the preparation of the given sa...
Text Solution
|
- Write balanced chemical equations, for the preparation of the given sa...
Text Solution
|
- Name the element An alkaline earth metal present in group 2 and peri...
Text Solution
|
- Name the element A trivalent metal used to make light tools.
Text Solution
|
- Name the element A monovalent non-metal present in fluorspar.
Text Solution
|
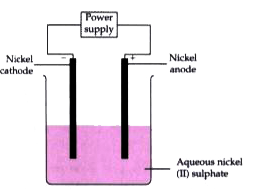
- An aqueous solution of nickel (II) sulphate was electrolyzed using nic...
Text Solution
|
- An aqueous solution of nickel (II) sulphate was electrolyzed using nic...
Text Solution
|
- An aqueous solution of nickel (II) sulphate was electrolyzed using nic...
Text Solution
|