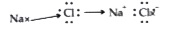Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE PAPER 2019-SECTION-II (40 Marks) Attempt any four questions from this Section
- Draw the electron dot structure of : Nitrogen molecule (N=7]
Text Solution
|
- Explain with the help of [i] an ionic equation [ii] electron do...
Text Solution
|
- Draw an electron dot diagram to show the formation of ammonium ion [N ...
Text Solution
|
- The pH values of three solution, A, B and C are given in the table. An...
Text Solution
|
- The pH values of three solution, A, B and C are given in the table. An...
Text Solution
|
- The pH values of three solution, A, B and C are given in the table. An...
Text Solution
|
- Study the extract of the Periodic Table given below and answer the que...
Text Solution
|
- Study the extract of the Periodic Table given below and answer the que...
Text Solution
|
- Study the extract of the Periodic Table given below and answer the que...
Text Solution
|
- Study the extract of the Periodic Table given below and answer the que...
Text Solution
|
- The particles present in strong electrolytes are
Text Solution
|
- Name the particles present in Non-electrolyte
Text Solution
|
- The particles present in strong electrolytes are
Text Solution
|
- Distinguish between the following pairs of compounds using the reagent...
Text Solution
|
- Distinguish between the pairs of compounds using the reagent given in ...
Text Solution
|
- Distinguish between the following pairs of compounds using the reagent...
Text Solution
|
- Choose the method of preparation of the following salts, from the meth...
Text Solution
|
- Choose the method of preparation of the following salts, from the meth...
Text Solution
|
- Choose the method of preparation of the following salts, from the meth...
Text Solution
|
- Choose the method of preparation of the following salts, from the meth...
Text Solution
|