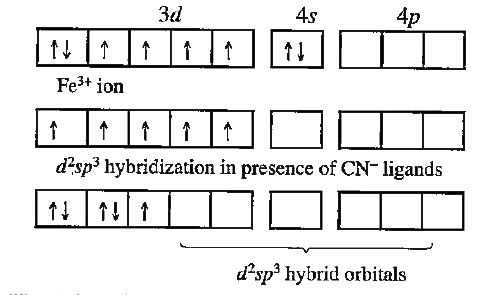Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE PAPER 2011-PART-II(SECTION-B)
- Give the IUPAC names for the following : (i) Na(3)[AlF(6)] (ii) [C...
Text Solution
|
- Give the IUPAC names of the following compound [Co(NH3)6]Cl3
Text Solution
|
- For the complex ion of [Fe(CN)6]^(3-) : Show the hybridization diagr...
Text Solution
|
- For the complex ion of [Fe(CN)6]^(3-) : Is it an inner orbital compl...
Text Solution
|
- For the complex ion of [Fe(CN)6]^(3-) : State its magnetic property.
Text Solution
|
- Give balanced chemical equations for the following: Chlorine gas i...
Text Solution
|
- Give balanced chemical equations for the following: Sulphur dioxide...
Text Solution
|
- Give balanced chemical equations for the following: Zinc is added ...
Text Solution
|
- Iron is ferromagnetic in nature. Explain why.
Text Solution
|
- The most common oxidation state exhibited by lanthanoids and actinoids...
Text Solution
|
- State the common oxidation state of : Actinides
Text Solution
|
- In a given transition series, there is no signifiecant change in the a...
Text Solution
|
- Give reactions and the conditions required for preparation of the foll...
Text Solution
|
- Give reactions and the conditions required for preparation of the foll...
Text Solution
|
- Carry out the following conversions : Methyl chloride to acetic aci...
Text Solution
|
- Carry out the following conversions : Benzene to benzoic acid.
Text Solution
|
- Carry out the following conversions : Ethanol to acetone.
Text Solution
|
- Deficiency of what vitamins will cause the following diseases : Nig...
Text Solution
|
- The deficiency of which vitamin will cause the following diseases: S...
Text Solution
|
- Give balanced equations for the following: Glycerol is heated with...
Text Solution
|