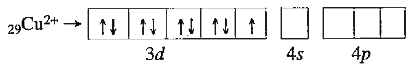Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE PAPER 2013-PART-II (SECTION-B)
- Give the IUPAC names of the following coordina tion compounds : K2[Z...
Text Solution
|
- Give the IUPAC names of the following coordina tion compounds : [CO(...
Text Solution
|
- For the complex ion [Fe(CN)6]^(3-) state The geometry of the ion.
Text Solution
|
- For the complex ion of [Fe(CN)6]^(3-) : State its magnetic property.
Text Solution
|
- What type of structural isouters are [Co(NH3)5Br]SO4 and Co[(NH3)5SO4]...
Text Solution
|
- For the molecule XeF2 : Draw the structure of the molecule indicati...
Text Solution
|
- For the molecule XeF2 : State the hybridisation of the central atom...
Text Solution
|
- For the molecule XeF2 : Draw the structure of the molecule indicati...
Text Solution
|
- Give balanced chemical equations for the following reactions : Fluor...
Text Solution
|
- Give balanced chemical equations for the following reactions : Hydro...
Text Solution
|
- Give balanced chemical equation for the reaction when potassium iodide...
Text Solution
|
- In the extraction of zinc from zinc blende: Give an equation to show...
Text Solution
|
- In the extraction of zinc from zinc blende: Give an equation to show...
Text Solution
|
- Explain why: (i) Transition elements form coloured compounds. (ii) C...
Text Solution
|
- Interhalogen compounds are more reactive than the individual halogens ...
Text Solution
|
- Explain why: Cu^(+) " is diamagnetic but " Cu^(2+) is paramag netic...
Text Solution
|