Text Solution
Verified by Experts
Topper's Solved these Questions
SAMPLE PAPER 2014
ICSE|Exercise PART-II (SECTION -B)|15 VideosSAMPLE PAPER 2014
ICSE|Exercise PART-II (SECTION -C)|21 VideosSAMPLE PAPER 2014
ICSE|Exercise PART-II (SECTION -C)|21 VideosSAMPLE PAPER 2013
ICSE|Exercise PART-II (SECTION-C)|17 VideosSAMPLE PAPER 2015
ICSE|Exercise PART-II SECTION-C QUESTION 10. |7 Videos
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE PAPER 2014-PART-II (SECTION -A)
- Write the mathematical expression relating the variation of rate const...
Text Solution
|
- How can you graphically find the activation energy of the reaction fro...
Text Solution
|
- The slope of the line in the graph of log k (k = rate constant) versus...
Text Solution
|
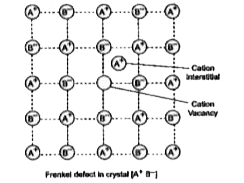
- Define Frenkel defect in solid crystal.
Text Solution
|
- Explain giving reasons why ionic solids conduct electricity in molten ...
Text Solution
|
- Explain giving reasons why : Solution of sodium chloride has no effec...
Text Solution
|
- For a crystal of diamond, state : The number of carbon atoms present...
Text Solution
|
- For a crystal of diamond, state : The type of lattice in which it cr...
Text Solution
|
- In a crystal of diamond : How many carbon atoms surround each carbon...
Text Solution
|
- In a crystal of diamond : How many carbon atoms surround each carbon...
Text Solution
|
- What is standard hydrogen electrode ? Give the reaction that occurs a...
Text Solution
|
- 0.05 "M" NaOH solution offered a resistance of 31.6 ohm in a conductiv...
Text Solution
|
- Kc for the reaction SO2(g) + 1/2 O(2(g)) to SO(3(g)) " is " 61*7 " at ...
Text Solution
|
- What happens to the equilibrium in a reversi ble reaction if a catalys...
Text Solution
|
- State the effect of the following on the reaction 2SO(2(g)) + O(2(g)) ...
Text Solution
|
- State the effect of the following on the reaction 2SO(2(g)) + O(2(g)) ...
Text Solution
|
- State the effect of the following on the reaction 2SO(2(g)) + O(2(g)) ...
Text Solution
|
- State the effect of the following on the reaction 2SO(2(g)) + O(2(g)) ...
Text Solution
|
- 0*3605 g of a metal is deposited on the electrode by passing 1*2 amper...
Text Solution
|
- Explain why phenolphthalein is used as an indicator in acid-base titra...
Text Solution
|