Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE QUESTION PAPER -4-SECTION - II
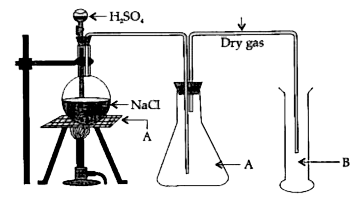
- With respect to the laboratory preparation of hydrochloric acid ans...
Text Solution
|
- In the laboratory preparation of hydrochloric acid, HCl gas is dissolv...
Text Solution
|
- With respect to the laboratory preparation of hydrochloric acid ans...
Text Solution
|
- Name the method used for obtaining ammonia on a large scale .
Text Solution
|
- What is the actual ratio of the reactants ?
Text Solution
|
- The temperature used is 450^(@)C . Explain why ? (A) A lower tempe...
Text Solution
|
- Write the reaction of sodium with water.
Text Solution
|
- An element X belongs to 3rd period and group II of the periodic tabl...
Text Solution
|
- An element X belongs to 3rd period and group II of the periodic tabl...
Text Solution
|
- An element X belongs to 3rd period and group II of the periodic tabl...
Text Solution
|
- An element X belongs to 3rd period and group II of the periodic tabl...
Text Solution
|
- State modern periodic law of classification of elements.
Text Solution
|
- Atomic size increases down a group of the periodic table. Explain.
Text Solution
|
- Give reason why ? People suffering from acidity are advised to dr...
Text Solution
|
- Give reasons why : Sodium chloride will conduct electricity only in...
Text Solution
|
- Give reason why ? Anhydrous HCl is a poor conductor while aqueous ...
Text Solution
|
- Give reasons for the following : (i) Methane does not undergo addit...
Text Solution
|
- Write balanced equation for the following: (i) Preparation of ethane...
Text Solution
|
- Write the equation for the ethene from iodoethane
Text Solution
|
- Write the equation for the laboratory preparation of ethyne (acetylene...
Text Solution
|