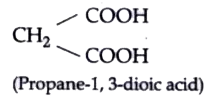Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SELF ASSESSMENT PAPER 03-Questions
- Differentiate between inductive and electromeric effect
Text Solution
|
- Discuss how the valence bond theory explains the pyramidal shape of NH...
Text Solution
|
- Balance the following equations by oxidation number method. MnO(2)+H...
Text Solution
|
- Calculate the oxidation number of the underlined atom in the following...
Text Solution
|
- Calculate the oxidation number of the underlined atom in the following...
Text Solution
|
- Calculate the oxidation number of the underlined atom in the following...
Text Solution
|
- Calculate the oxidation number of the underlined atom in the following...
Text Solution
|
- Balance the following equations by ion electron method. AsO(3)^(3-)...
Text Solution
|
- Calculate the oxidation number of the underlined atom in the following...
Text Solution
|
- Calculate the oxidation number of the underlined atom in the following...
Text Solution
|
- Calculate the oxidation number of the underlined atom in the following...
Text Solution
|
- Calculate the oxidation number of the underlined atom in the following...
Text Solution
|
- An acid of molecular mass 104 contains 34.6% carbon and 3.85% hydrogen...
Text Solution
|
- A hydrocarbon (A) containing 90% carbon and having V.D. 20 reacts with...
Text Solution
|
- At 20 ^(@) C the solubility of N2 gas in water is 0.0150 g L^(-1) ...
Text Solution
|
- Calculate the degree of ionisation and (H3O^(+) ) of a 0.15 M CH3COOH ...
Text Solution
|
- The hydronium lon concentration of a fruit juice is 4.6 xx 10^(-4) " m...
Text Solution
|
- A liquid is in equilibrium with its vapour in a seated container at a ...
Text Solution
|
- A liquid is in equilibrium with its vapour in a seated container at a ...
Text Solution
|
- A liquid is in equilibrium with its vapour in a seated container at a ...
Text Solution
|