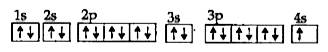Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE QUESTION PAPER 03-Questions
- How many electrons are unpaired in C
Text Solution
|
- How many electrons are unpaired in N
Text Solution
|
- How many electrons are unpaired in K
Text Solution
|
- At room temperature, ammonia gas at 1 atm pressure and hydrogen chlori...
Text Solution
|
- A 4: 1 molar mixture of He and CH4 is contained in a vessel at 20 bar ...
Text Solution
|
- Write the balance equation for the following Action of heat on Na(2...
Text Solution
|
- Write a balanaed chemical equation for each of the following: Actio...
Text Solution
|
- Calculate the standard free energy change for the following reaction a...
Text Solution
|
- Define the term standard free energy change (Delta G^(@)). How is it r...
Text Solution
|
- Comment on the spontaneity of a process when DeltaH lt 0, T Delta S ...
Text Solution
|
- Comment on the spontaneity of a process when Delta H gt 0, T Delta S...
Text Solution
|
- Comment on the spontaneity of a process when DeltaH gt 0, T Delta Sgt...
Text Solution
|
- Comment on the spontaneity of a process when DeltaH lt 0, T Delta S ...
Text Solution
|
- What is smog and how it is formed?
Text Solution
|
- Define metamerism. What type of compounds do show it? Give an example.
Text Solution
|
- Discuss the shape of the B Cl(3) molecules using VSEPR model .
Text Solution
|
- On the basis of VSEPR theory, predict the shapes of the following mole...
Text Solution
|
- Dicuss the shape of the following molecules using the VSEPR model: A...
Text Solution
|
- Justify that the following reactions are redox reactions : CuO(s)+H(...
Text Solution
|
- Justify that the following reactions are redox reactions : Fe(2)O(3)...
Text Solution
|