Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE QUESTION PAPER 03-Questions
- What is smog and how it is formed?
Text Solution
|
- Define metamerism. What type of compounds do show it? Give an example.
Text Solution
|
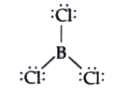
- Discuss the shape of the B Cl(3) molecules using VSEPR model .
Text Solution
|
- On the basis of VSEPR theory, predict the shapes of the following mole...
Text Solution
|
- Dicuss the shape of the following molecules using the VSEPR model: A...
Text Solution
|
- Justify that the following reactions are redox reactions : CuO(s)+H(...
Text Solution
|
- Justify that the following reactions are redox reactions : Fe(2)O(3)...
Text Solution
|
- Justify that the following reactions are redox reactions : 4BCl(3)(g...
Text Solution
|
- Write formulase for the following compounds : Mg(II) chloride
Text Solution
|
- Write formulas for the following compounds : Nickel (II) sulphate
Text Solution
|
- Write formulas for the following compounds : Tin (IV) oxide
Text Solution
|
- Write formulas for the following compounds : Thallium (I) sulphate
Text Solution
|
- Identify the substance oxidised and reduced, oxidising agent and reduc...
Text Solution
|
- Calculate the oxidation number of sulphur , chromium and nitrogen in H...
Text Solution
|
- Explain why A branched chain alkane possesses lower boiling point than...
Text Solution
|
- Why do alkenes and alkynes undergo addition reactions ? Describe some ...
Text Solution
|
- How will you convert benzene into (i) p-nitrobromobenzene (ii) m-...
Text Solution
|
- How will you convert the following: (Give balanced equation) Ethyne...
Text Solution
|
- How will you bring out the following conversions ? Ethene to ethyn...
Text Solution
|
- An alkene ‘A’ contains three C – C, eight C – H (sigma) bonds and one...
Text Solution
|