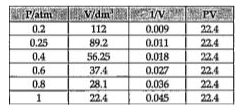
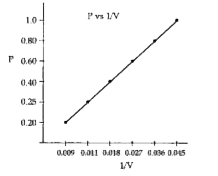
Text Solution
Verified by Experts
Topper's Solved these Questions
STUDY OF GAS LAWS
ICSE|Exercise TOPIC 2 (1 Mark Questions ) |10 VideosSTUDY OF GAS LAWS
ICSE|Exercise TOPIC 2 (2 Marks Questions ) |27 VideosSTUDY OF GAS LAWS
ICSE|Exercise TOPIC 1 (3 Marks Questions ) |17 VideosSPECIMEN PAPER 3
ICSE|Exercise SECTION II|35 VideosSTUDY OF THE FIRST ELEMENT - HYDROGEN
ICSE|Exercise TOPIC 2 Properties of Hydrogen, Oxidation nd Reduction (5 MARKS QUESTIONS)|29 Videos
Similar Questions
Explore conceptually related problems
ICSE-STUDY OF GAS LAWS -TOPIC 1 (5 Marks Questions )
- State five important assumptions of the kinetic theory of matter.
Text Solution
|
- See the given figure below and answer the given questions? What doe...
Text Solution
|
- See the given figure below and answer the given questions? Write th...
Text Solution
|
- See the given figure below and answer the given questions? What are...
Text Solution
|
- Boyle.s Law is
Text Solution
|
- Give the significanceof Boyle.s law
Text Solution
|
- A student performed an experiment to measure pressure and volume of a ...
Text Solution
|
- At constant temperature , the effect of change of pressure on volume o...
Text Solution
|
- At constant temperature , the effect of change of pressure on volume o...
Text Solution
|
- At constant temperature, the effect of change of pressure on volume of...
Text Solution
|