Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-FLUIDS-TOPIC 2 (4 Marks Questions )
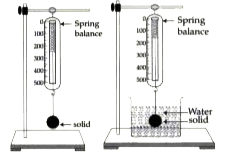
- A metal piece weighs 200gf in air and 150 gf when completely immersed ...
Text Solution
|
- A metal piece weighs 200gf in air and 150 gf when completely immersed ...
Text Solution
|
- A block of wood of volume 30 "cm"^3 floats it water with 20 "cm"^3 of ...
Text Solution
|
- A block of wood of volume 30 "cm"^3 floats in water with 20 "cm"^3 of ...
Text Solution
|
- A solid weighs 32 gf in air and 28.8 gf in water. Find the volume of...
Text Solution
|
- A solid weighs 32 gf in air and 28.8 gf in water. Find R.D. of solid
Text Solution
|
- A solid weighs 32 gf in air and 28.8 gf in water. Find the weight of...
Text Solution
|
- With the use of Archimedes' Principle, state how you will find relativ...
Text Solution
|
- A piece of stone of mass 15.1 g is first immersed in a liquid and it w...
Text Solution
|
- A piece of stone of mass 15.1 g is first immersed in a liquid and it w...
Text Solution
|
- A piece of stone of mass 15.1 g is first immersed in a liquid and it w...
Text Solution
|
- A piece of stone of mass 15.1 g is first immersed in a liquid and it w...
Text Solution
|
- A piece of wood of uniform cross section and height 15 cm floats verti...
Text Solution
|
- A wooden block floats in water with two third of its volume submerged....
Text Solution
|
- A wooden block floats in water with two-third of its volume submerged...
Text Solution
|
- The density of ice is 0.92 "g cm"^(-3) and that of sea water is 1.025...
Text Solution
|