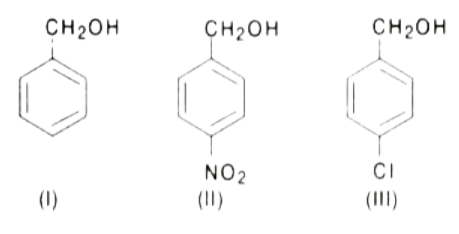
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
ICSE|Exercise EXERCISE (PART-I Objective Questions) (Correct the following statements)|15 VideosALCOHOLS, PHENOLS AND ETHERS
ICSE|Exercise EXERCISE (PART-II Descriptive Questions) (VERY SHORT ANSWER QUESTIONS) |104 VideosALCOHOLS, PHENOLS AND ETHERS
ICSE|Exercise EXERCISE (PART-I Objective Questions) |25 VideosALCOHOLS , PHENOLS AND ETHERS
ICSE|Exercise SOURCE BASED QUESTIONS|4 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
ICSE|Exercise EXERCISE (PART-II DESCRIPTIVE QUESTIONS) ( LONG ANSWER QUESTIONS)|478 Videos
Similar Questions
Explore conceptually related problems
ICSE-ALCOHOLS, PHENOLS AND ETHERS -EXERCISE (PART-I Objective Questions) (Choose the correct alternative)
- Which of the following is most acidic ?
Text Solution
|
- Mark the correct order of decreasing acid strength of the following co...
Text Solution
|
- Mark the correct increasing order of reactivity of the following compo...
Text Solution
|
- Arrange the following compounds in increasing order of boiling point. ...
Text Solution
|
- The reaction C(2)H(5)ONa +C(2)H(5)I to C(2)H(5)OC(2)H(5)+Nal is called
Text Solution
|
- Diethyl ether on heating with excess of HI yields
Text Solution
|
- When diethyl ether is heated with conc. sulfuric acid under pressure,...
Text Solution
|
- When vapours of ethyl alcohol are passed over Al(2)O(3) at 250^(@)C,...
Text Solution
|
- Tert. butyl chloride on treatment with sodium alkoxide yields
Text Solution
|
- Diethyl ether on treatment with Cl(2) in presence of sunlight gives
Text Solution
|
- Complete combustion of ether gives?
Text Solution
|
- Which of the following pairs of reagents will not form ether?
Text Solution
|
- According to Lewis concept of acids and bases, ethers are
Text Solution
|
- Oxygen atom of ether is
Text Solution
|
- Williamson synthesis is used to prepare
Text Solution
|
- Sometimes explosion may occur while distilling ether. It may be due t...
Text Solution
|
- Diethyl ether is soluble in
Text Solution
|
- Ethoxyethane does not react with
Text Solution
|
- Which of the following statement is correct?
Text Solution
|
- Intermolecular hydrogen bonds are not present in ?
Text Solution
|