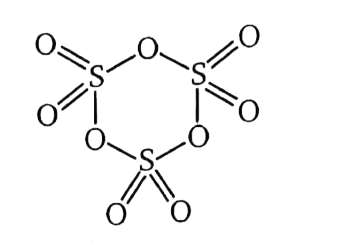
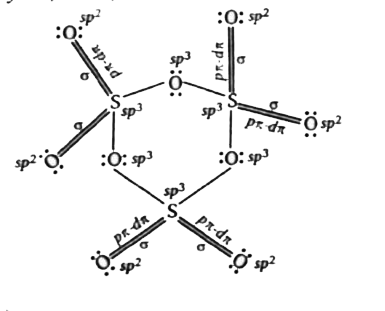
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VK JAISWAL ENGLISH-CHEMICAL BONDING (ADVANCED)-SUBJECTIVE PROBLEMS
- Calculate value of |x-y|, here x and y are the total number of bonds i...
Text Solution
|
- Consider the following compounds: (i)IF(5) (ii)ClI(4)^(-) (iii)XeO...
Text Solution
|
- Consider the following Calculate value of p+q, here p and q are tot...
Text Solution
|
- Consider the following orbitals (i)3p(x) (ii)4d(z^(2)) (iii)3d(x^(2)...
Text Solution
|
- Consider the following oxyanions: PO(4)^(3-),P(2)O(6)^(4-),SO(4)^(2-...
Text Solution
|
- Consider the following three compounds (i)AX(2n)^(n-), (ii)AX(3n) and...
Text Solution
|
- Consider the following compounds (1)H(3)CF (2)H(2)CF(2) (3) CH(4) ...
Text Solution
|
- There are some species given below. (i)O(2)^(+) (ii)CO (iii)B(2) ...
Text Solution
|
- Following compounds A and B have similar structure with delocalization...
Text Solution
|
- The hybridization of central atoms of compounds A, B, C and D are sp^(...
Text Solution
|
- In compound PCl(x)F(5-x), possible values of x are 0 to 5, then calcul...
Text Solution
|
- There are two groups of compounds A and B. Groups A contains three com...
Text Solution
|
- Consider the following three compounds (i)AX(2n)^(n-), (ii)AX(3n) and...
Text Solution
|
- When B(2)H(4) is allowed to react with following lewis bases, then how...
Text Solution
|
- Consider the following elements A, B, C and D and their outer electron...
Text Solution
|
- Consider following four compounds: (i) C(x) O(y) (ii) C(x)O(y+1) ...
Text Solution
|
- Calculate expression (x+y+z) for diatomic molecules. where x=Total n...
Text Solution
|
- If Hund rule violate, then find the total number of species among foll...
Text Solution
|
- Consider the following table Than calculate value of experssion...
Text Solution
|
- Total number of species among following, in which bond angle is equal...
Text Solution
|