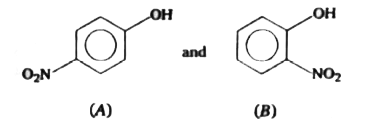
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VK JAISWAL ENGLISH-CHEMICAL BONDING (ADVANCED)-SUBJECTIVE PROBLEMS
- Out of the two compounds shown below, the vapour pressure of B at a pa...
Text Solution
|
- There are two groups of compounds A and B. Groups A contains three com...
Text Solution
|
- Consider the following three compounds (i)AX(2n)^(n-), (ii)AX(3n) and...
Text Solution
|
- When B(2)H(4) is allowed to react with following lewis bases, then how...
Text Solution
|
- Consider the following elements A, B, C and D and their outer electron...
Text Solution
|
- Consider following four compounds: (i) C(x) O(y) (ii) C(x)O(y+1) ...
Text Solution
|
- Calculate expression (x+y+z) for diatomic molecules. where x=Total n...
Text Solution
|
- If Hund rule violate, then find the total number of species among foll...
Text Solution
|
- Consider the following table Than calculate value of experssion...
Text Solution
|
- Total number of species among following, in which bond angle is equal...
Text Solution
|
- Total number of unpaired electrons(s) present in both cationic and an...
Text Solution
|
- Total number of species which has/ have symmetrical electronic distrib...
Text Solution
|
- Total number of molecules, in which each covalent bond is comprised of...
Text Solution
|
- Total number of angle in SeCl(4) which are less than 90^(@).
Text Solution
|
- Consider the following species O(Me)(2), N(SiH(3))(3), CO, O(SiH(3))(2...
Text Solution
|
- Total number of molecules which can form H-bond among themselves. Si...
Text Solution
|
- Consider two covalent compounds AL(n(1)) and BL(n(2)), if central atom...
Text Solution
|
- Calculate the I-I distance in (Å) for given compound H(2)C(2) I(2) if ...
Text Solution
|
- There are some arrangements of atomic orbitals which are given below: ...
Text Solution
|
- Number of hybrid orbital C atoms which have 33% p-character in C(CN)(4...
Text Solution
|
- Max. no. of equal P-O bonds in P(2)O(7)^(4-) ion is :
Text Solution
|