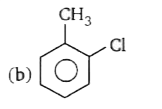
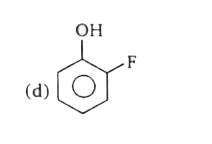
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING (ADVANCED)
VK JAISWAL ENGLISH|Exercise MATCH THE COLUMN|21 VideosCHEMICAL BONDING (ADVANCED)
VK JAISWAL ENGLISH|Exercise ASSERTION-REASON TYPE QUESTIONS|57 VideosCHEMICAL BONDING (ADVANCED)
VK JAISWAL ENGLISH|Exercise Level 3|77 VideosCHEMICAL BONDING (BASIC)
VK JAISWAL ENGLISH|Exercise SUBJECTIVE PROBLEMS|54 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL ENGLISH-CHEMICAL BONDING (ADVANCED)-ONE OR MORE ANSWER IS/ARE CORRECT
- The correct statement(s) is /are :
Text Solution
|
- Consider the following reversible chemical reactions : A(2)(g)+B(2)...
Text Solution
|
- In which of the following molecules mu(exp) (observed dipole moment) i...
Text Solution
|
- Correct statement (s) regarding As(CH(3))F(2)Cl(2) molecule is/are:
Text Solution
|
- Which of the following species are involved
Text Solution
|
- Which of the following statements is correct?
Text Solution
|
- Which of the following species is/are not know?
Text Solution
|
- Select correct order between following compounds:
Text Solution
|
- Which of the following is (are) V-shaped?
Text Solution
|
- Which of the following equlibria would have highest and lowest value o...
Text Solution
|
- Which of the following process is/are assciated with change of hybridi...
Text Solution
|
- Which of the following are true?
Text Solution
|
- Which of the following statement is incorrect?
Text Solution
|
- Which of the following statements are not correct?
Text Solution
|
- In the structure of H(2)CSF(4), which of the following statement is/ar...
Text Solution
|
- In which compound compounds vacant hybride orbitals take part in bondi...
Text Solution
|
- Which of the following is true for N(2)O?
Text Solution
|
- Silane is more reactive than CH(4) towards Nu^(-) substitution due to...
Text Solution
|
- Which of the following statement(s) is/are not correct for following...
Text Solution
|
- If N(B) is the number of bonding electron and N(A) is the number of an...
Text Solution
|