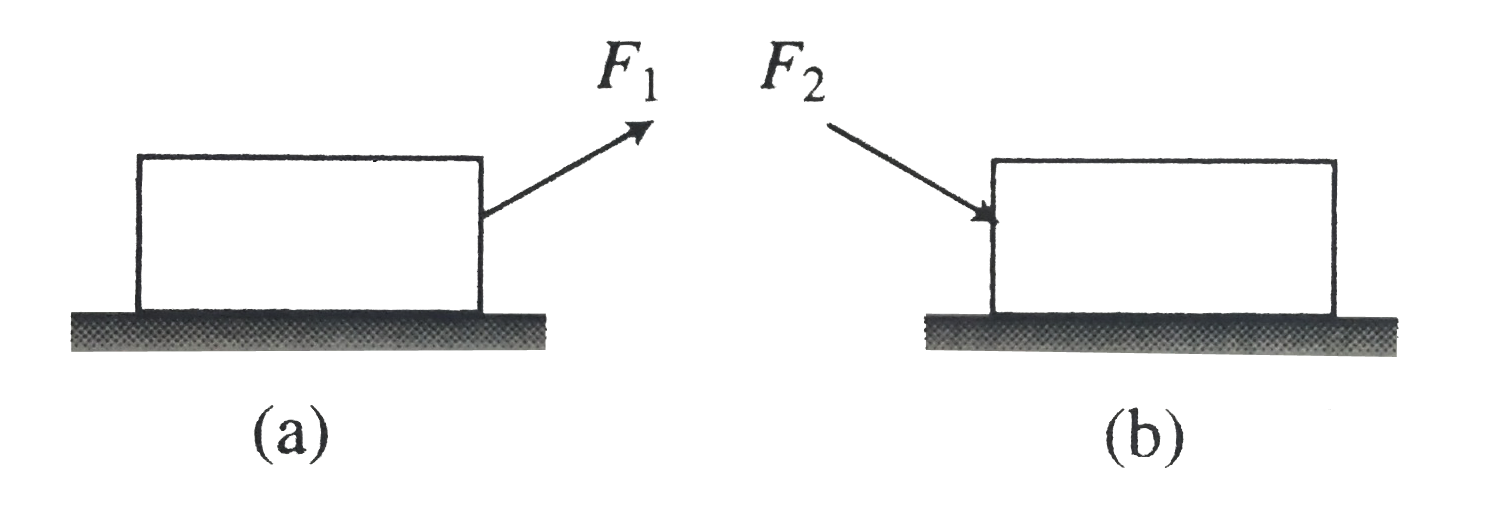
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NEWTON'S LAWS OF MOTION 1
CENGAGE PHYSICS ENGLISH|Exercise Linked Comperhension|46 VideosNEWTON'S LAWS OF MOTION 1
CENGAGE PHYSICS ENGLISH|Exercise Integer|5 VideosNEWTON'S LAWS OF MOTION 1
CENGAGE PHYSICS ENGLISH|Exercise Multiple Correct|11 VideosMISCELLANEOUS VOLUME 2
CENGAGE PHYSICS ENGLISH|Exercise INTEGER_TYPE|10 VideosNEWTON'S LAWS OF MOTION 2
CENGAGE PHYSICS ENGLISH|Exercise Integer type|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS ENGLISH-NEWTON'S LAWS OF MOTION 1-Assertion-reasoning
- Statement I: The driver of a moving car sees a wall in front of him. T...
Text Solution
|
- Statement I: A block of mass m is placed on a smooth inclined plane of...
Text Solution
|
- Statement I: A particle is found to be a rest when seen from a frame S...
Text Solution
|
- Statement I: The coefficient of friction can be greater than unity. ...
Text Solution
|
- Statement I: In high jump, it hurts less when an athlete lands on a he...
Text Solution
|
- Statement I: A body in equilibrium has to be at reat only. Statement...
Text Solution
|
- Statement I: Pulling is easier than pushing on a rough surface. ...
Text Solution
|
- Statement I: A block is lying stationary as on inclined plane and coef...
Text Solution
|
- Statement I: Two particle are moving towards each other due to mutual ...
Text Solution
|
- Statement I: A concept of pseudo force is valid both for inertial as w...
Text Solution
|
- Statement I: In fig., ground is smooth and masses of both the block ar...
Text Solution
|
- Statement I: The greater the rate of the change in the momentum vector...
Text Solution
|
- Statement I: Frictional heat generated by the moving ski is the chief ...
Text Solution
|
- A relerence frame atteched to the Earth
Text Solution
|
- Statement I: Block A is moving on the horizontal surface towards right...
Text Solution
|